灵芝经皮化合物 (±)-spiroapplanatumine O 和 (±)-applanatumol B/W 的合成
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
(±)-applanatumol W 的全合成、(±)-spiroapplanatumine O 和 (±)-applanatumol B 的正式合成都是使用一种常见的中间体--甲基苯甲酰环戊烷羧酸酯 9 来完成的。这种中间体是通过单锅反应合成的,其中包括首先通过丙二酸与丙烯醛的迈克尔加成反应、醛缩合和脱水形成迈克尔受体环戊烯酮,然后通过烯丙基硫酸酯的迈克尔加成反应和随后的脱甲氧基羧化反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
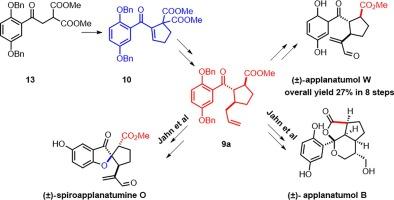
Syntheses of Ganoderma meroterpenoids (±)-spiroapplanatumine O and (±)-applanatumol B/W
Total synthesis of (±)-applanatumol W, formal syntheses of (±)-spiroapplanatumine O and (±)-applanatumol B were accomplished using a common intermediate, methyl benzoyl cyclopentane carboxylate 9. This intermediate was synthesized through a one-pot reaction involving formation of Michael acceptor cyclopentenone through first Michael addition of malonate to acrolein, Aldol condensation and dehydration, followed by Michael addition of allylcuprate and subsequent demethoxycarboxylation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


