使用 RedEx 方法对复杂的克隆 DNA 序列进行无缝定点诱变。
IF 13.1
1区 生物学
Q1 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
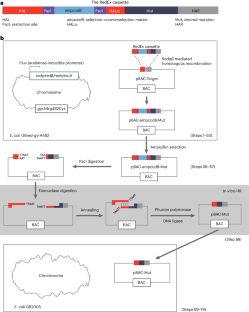
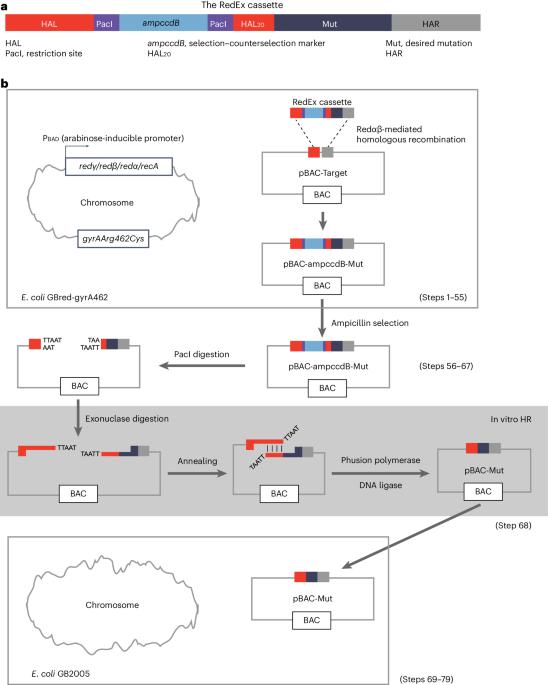
无缝定点诱变是研究蛋白质功能、调整酶催化活性和多轮修饰遗传元件的重要技术,因为它可以在目标位点插入、删除或替换核苷酸、DNA片段甚至整个基因,而不会引入任何不必要的变化。为了便于在具有重复序列的大型质粒和细菌人工染色体(BAC)上进行无缝定点诱变,我们最近开发了 RedEx 策略。与以前的方法相比,我们的方法通过规避重复序列之间不必要的重组,实现了正确重组子的高准确率回收。由于在编码几乎相同酶的模块中存在大量重复 DNA 序列,因此这些基因簇是最难处理的目标之一。在这里,我们详细介绍了在 BAC 载体中进行无缝定点诱变的 RedEx 方法。总的来说,该过程包括三个部分:(1)通过重组将含有所需突变的 RedEx 盒以及两侧有独特限制性位点和 20-bp 重叠序列的选择-反选标记插入目标位点;(2)通过限制性消化去除 BAC 中的选择-反选标记;(3)通过外切酶介导的体外 DNA 退火将线性 BAC 环化。该方案可在 3 周内完成,可使具有 DNA 克隆经验的研究人员掌握无缝定点诱变技术,加快研究进度。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Seamless site-directed mutagenesis in complex cloned DNA sequences using the RedEx method
Seamless site-directed mutagenesis is an important technique for studying protein functions, tuning enzyme catalytic activities and modifying genetic elements in multiple rounds because it can insert, delete or substitute nucleotides, DNA segments or even entire genes at the target site without introducing any unwanted change. To facilitate seamless site-directed mutagenesis in large plasmids and bacterial artificial chromosomes (BACs) with repetitive sequences, we recently developed the RedEx strategy. Compared with previous methods, our approach achieves the recovery of correct recombinants with high accuracy by circumventing unwanted recombination between repetitive sequences. RedEx readily yields more than 80% accuracy in seamless DNA insertion and deletion in large multimodular polyketide synthase gene clusters, which are among the most difficult targets due to the large number of repetitive DNA sequences in modules encoding almost identical enzymes. Here we present the RedEx method by describing in detail the seamless site-directed mutagenesis in a BAC vector. Overall, the process includes three parts: (1) insertion of the RedEx cassette containing the desired mutation together with selection–counterselection markers flanked by unique restriction sites and 20-bp overlapping sequences into the target site by recombineering, (2) removal of the selection–counterselection markers in the BAC by restriction digestion and (3) circularization of the linear BAC by exonuclease-mediated in vitro DNA annealing. This protocol can be performed within 3 weeks and will enable researchers with DNA cloning experience to master seamless site-directed mutagenesis to accelerate their research. RedEx achieves seamless genetic modification of large, highly repetitive, DNA targets by combining Redαβ-mediated linear–circular homologous recombination, ccdB counterselection and exonuclease-mediated in vitro annealing.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Protocols
生物-生化研究方法
CiteScore
29.10
自引率
0.70%
发文量
128
审稿时长
4 months
期刊介绍:
Nature Protocols focuses on publishing protocols used to address significant biological and biomedical science research questions, including methods grounded in physics and chemistry with practical applications to biological problems. The journal caters to a primary audience of research scientists and, as such, exclusively publishes protocols with research applications. Protocols primarily aimed at influencing patient management and treatment decisions are not featured.
The specific techniques covered encompass a wide range, including but not limited to: Biochemistry, Cell biology, Cell culture, Chemical modification, Computational biology, Developmental biology, Epigenomics, Genetic analysis, Genetic modification, Genomics, Imaging, Immunology, Isolation, purification, and separation, Lipidomics, Metabolomics, Microbiology, Model organisms, Nanotechnology, Neuroscience, Nucleic-acid-based molecular biology, Pharmacology, Plant biology, Protein analysis, Proteomics, Spectroscopy, Structural biology, Synthetic chemistry, Tissue culture, Toxicology, and Virology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


