分子对接电解质实现高压锂电池化学反应
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
理想的可充电锂电池电解质应能促进电极表面附近的法拉第反应,同时减轻不希望发生的副反应。然而,传统电解质由于解溶能量高、兼容性差,通常会表现出缓慢的动力学和严重的降解。在此,我们提出了一种电解质设计策略,该策略克服了锂盐在非配位溶剂中解离的相关限制,从而实现了快速、稳定的锂化学反应。当非配位溶剂与含氟苯或卤代烷烃化合物混合时,可通过有利的氢键相互作用(特别是 Fδ--Hδ+ 或 Hδ+-Oδ- )激活非配位溶剂。这些分子间相互作用实现了动态的 Li+ 溶剂配位过程,从而促进了快速的 Li+ 反应动力学并抑制了电极副反应。利用这种分子对接电解质设计策略,我们开发出了 25 种电解质,它们在全电池和袋式电池中均表现出较高的锂镀/剥离库仑效率和良好的容量保持能力。这项工作支持利用分子对接溶解机制设计具有快速锂+动力学的电解质,用于高压锂电池。本文章由计算机程序翻译,如有差异,请以英文原文为准。
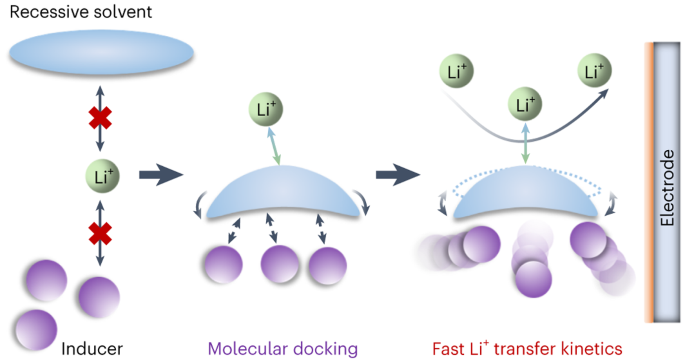
Molecular-docking electrolytes enable high-voltage lithium battery chemistries
Ideal rechargeable lithium battery electrolytes should promote the Faradaic reaction near the electrode surface while mitigating undesired side reactions. Yet, conventional electrolytes usually show sluggish kinetics and severe degradation due to their high desolvation energy and poor compatibility. Here we propose an electrolyte design strategy that overcomes the limitations associated with Li salt dissociation in non-coordinating solvents to enable fast, stable Li chemistries. The non-coordinating solvents are activated through favourable hydrogen bond interactions, specifically Fδ−–Hδ+ or Hδ+–Oδ−, when blended with fluorinated benzenes or halide alkane compounds. These intermolecular interactions enable a dynamic Li+–solvent coordination process, thereby promoting the fast Li+ reaction kinetics and suppressing electrode side reactions. Utilizing this molecular-docking electrolyte design strategy, we have developed 25 electrolytes that demonstrate high Li plating/stripping Coulombic efficiencies and promising capacity retentions in both full cells and pouch cells. This work supports the use of the molecular-docking solvation mechanism for designing electrolytes with fast Li+ kinetics for high-voltage Li batteries. Conventional Li-ion battery electrolytes often show sluggish kinetics and severe degradation due to high Li+ desolvation energies and poor compatibility. Now, a molecular-docking strategy between solvents and inducers has been shown to enable dynamic Li+ coordination that promotes fast, stable and high-voltage lithium battery chemistries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


