Vioprolide B 及其脱氢丁炔甘氨酸类似物的合成与生物学评价
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在本文中,我们介绍了全合成反肽类化合物 vioprolide B 及其类似物的方法,其中 (E)-dehydrobutyrine 氨基酸被甘氨酸取代。在生物试验中对这些化合物进行了研究,结果表明,vioprolide B 可能通过与延伸因子 eEF1A1 和染色质组装因子 CHAF1A 的半胱氨酸残基共价结合而具有细胞毒性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
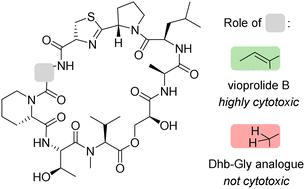
Synthesis and biological evaluation of vioprolide B and its dehydrobutyrine-glycine analogue†
Herein, we describe the total synthesis of the depsipeptide vioprolide B and of an analogue, in which the (E)-dehydrobutyrine amino acid was replaced by glycine. The compounds were studied in biological assays which revealed cytotoxicity solely for vioprolide B presumably by covalent binding to cysteine residues of elongation factor eEF1A1 and of chromatin assembly factor CHAF1A.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


