环状 RNA 寡核苷酸:酶法合成和纳米结构支架
IF 8
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
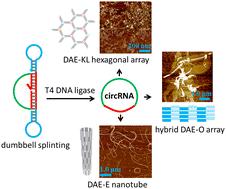
我们报告了利用一种新颖的 DNA 哑铃拼接加 T4 DNA 连接策略高效合成大小范围为 16-44 nt 的单体环状 RNA(circRNA)的情况。我们中的一个研究小组最近开发出了这种 DNA 哑铃拼接策略,可将短线性 DNA 近乎定量地转化为单体环状 DNA。此外,我们还以 44 nt circRNA 为支架链,构建了混合 RNA:DNA 和纯 RNA:RNA 双交叉瓦片及其核酸纳米管和扁平阵列组装体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Circular RNA oligonucleotides: enzymatic synthesis and scaffolding for nanoconstruction†
We report the efficient synthesis of monomeric circular RNAs (circRNAs) in the size range of 16–44 nt with a novel DNA dumbbell splinting plus T4 DNA ligation strategy. Such a DNA dumbbell splinting strategy was developed by one group among ours recently for near-quantitative conversion of short linear DNAs into monomeric circular ones. Furthermore, using the 44 nt circRNA as scaffold strands, we constructed hybrid RNA:DNA and pure RNA:RNA double crossover tiles and their assemblies of nucleic acid nanotubes and flat arrays.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nanoscale Horizons
Materials Science-General Materials Science
CiteScore
16.30
自引率
1.00%
发文量
141
期刊介绍:
Nanoscale Horizons stands out as a premier journal for publishing exceptionally high-quality and innovative nanoscience and nanotechnology. The emphasis lies on original research that introduces a new concept or a novel perspective (a conceptual advance), prioritizing this over reporting technological improvements. Nevertheless, outstanding articles showcasing truly groundbreaking developments, including record-breaking performance, may also find a place in the journal. Published work must be of substantial general interest to our broad and diverse readership across the nanoscience and nanotechnology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


