利用氢氧化四丁基铵合成单壁沸石纳米管的路线
IF 5.7
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
单壁沸石纳米管(ZNT)是最近在一个狭窄的成分窗口中合成的。ZNT 的结构特征--薄沸石壁和大介孔--可使小分子轻松进入沸石微孔,但也给这些材料的加工带来了限制,例如传统的水相离子交换条件。煅烧过的 NaOH 衍生 ZNT(NaH-ZNT)的传统固相和液相离子交换会导致结构退化为二维片状相、三维纳米晶体或无定形相,这促使人们采用不同的直接合成路线和非煅烧 ZNT 前体的非常规离子交换程序。本文介绍了一种经过改进的 ZNT 合成路线,该路线便于离子交换以及在沸石框架中加入额外的非铝杂质原子。使用四丁基氢氧化铵(TBAOH)作为氢氧化物源和共 OSDA,可以合成新成分的 ZNT,否则无法通过对以前报道过的 NaH-ZNT 进行后修饰来实现。通过改变凝胶成分、合成温度、结晶时间、氢氧化物源、硅源和铝源,开发出了新 TBAOH 合成的生产条件,从而增加了 ZNT 中的强酸位点密度。收集到的结果证明了 ZNT 合成对许多关键参数的敏感性,并表明在这些合成凝胶中,只有当 Si/(Al + T) ∼ 30 时,ZNT 才会形成,并使用特定的硅源和铝源,而且始终存在痕量 Na+。通过串联二氧化碳加氢制甲醇和甲醇制芳烃反应进行的催化测试表明,与传统的三维催化剂相比,ZNT 在将甲醇转化为芳烃化合物方面具有足够的催化活性(酸性)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
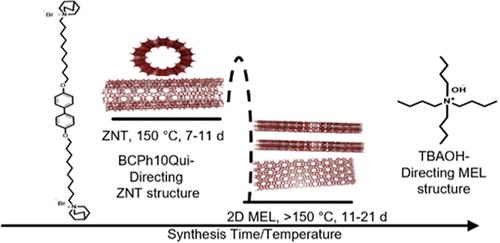
Synthesis Route to Single-Walled Zeolite Nanotubes Enabled by Tetrabutylammonium Hydroxide
Single-walled zeolite nanotubes (ZNT) were recently synthesized in a narrow compositional window. ZNT structural features─thin zeolitic walls and large mesopores─can allow for easy access of small molecules to zeolite micropores, but they also impart processing limitations for these materials, such as challenges with conventional aqueous ion-exchange conditions. Conventional solid- and liquid-phase ion exchange of calcined NaOH-derived ZNT (NaH-ZNT) results in structural degradation to either 2D sheet-like phases, 3D nanocrystals, or amorphous phases, motivating different direct synthesis routes and unconventional ion-exchange procedures of uncalcined ZNT precursors. Here, a modified synthesis route for ZNT synthesis is introduced that facilitates facile ion exchange as well as incorporation of additional non-Al heteroatoms in the zeolite framework. Tetrabutylammonium hydroxide (TBAOH) is used as a hydroxide source and co-OSDA, enabling synthesis of new compositions of ZNT, otherwise unachievable by post-modification of previously reported NaH-ZNT. By varying the gel composition, synthesis temperature, crystallization time, hydroxide source, silicon source, and aluminum source, productive conditions for the new TBAOH synthesis are developed, leading to increased strong acid site density in the ZNT. The collected results demonstrate the sensitivity of the ZNT synthesis to many key parameters and show that the ZNT forms only when Si/(Al + T) ∼ 30 in these synthesis gels and with specific Si and Al sources, and always in the presence of trace Na+. Catalytic testing, via the tandem CO2 hydrogenation to methanol and methanol to aromatics reaction, shows that ZNTs provide adequate catalytic activity (acidity), relative to their conventional 3D counterparts in converting methanol to aromatic compounds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Materials Au
材料科学-
CiteScore
5.00
自引率
0.00%
发文量
0
期刊介绍:
ACS Materials Au is an open access journal publishing letters articles reviews and perspectives describing high-quality research at the forefront of fundamental and applied research and at the interface between materials and other disciplines such as chemistry engineering and biology. Papers that showcase multidisciplinary and innovative materials research addressing global challenges are especially welcome. Areas of interest include but are not limited to:Design synthesis characterization and evaluation of forefront and emerging materialsUnderstanding structure property performance relationships and their underlying mechanismsDevelopment of materials for energy environmental biomedical electronic and catalytic applications
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


