介孔二氧化硅纳米粒子的表面改性是在三维肿瘤微环境中引入固有癌症靶向能力的一种手段
IF 11.1
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
介孔二氧化硅纳米颗粒(MSNs)是一种很有前景的药物载体,可促进抗癌药物的靶向递送,但依靠主动靶向机制进行的效率研究仍难以实现。本研究采用体外三维共培养物(即微组织)来模拟与生理相关的肿瘤微环境(TME),以研究不含靶向配体的表面修饰MSNs对肿瘤细胞和癌症相关成纤维细胞的内化、货物递送和货物释放的影响。其中,在含有胶原蛋白的三维环境中,乙酰化的MSN最有效地定位在肿瘤细胞中,而其他MSN的定位程度较低,这很可能是由于它们仍被困在TME的细胞外基质中。疏水性模型药物负载 MSN 的共聚焦成像显示,在二维和三维共培养中,货物主要在肿瘤细胞中有效释放。与游离形式的高疏水性药物相比,MSN介导的抗癌药物在微组织中的递送显著缩小了肿瘤器官大小,并增强了γ-分泌酶抑制剂的肿瘤特异性细胞毒性作用。这种固有的靶向潜力表明,可以减少脱靶效应,提高药物疗效,从而展示了 MSN 表面修饰作为一种直接细胞特异性靶向和给药手段,实现精确和成功靶向给药的前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
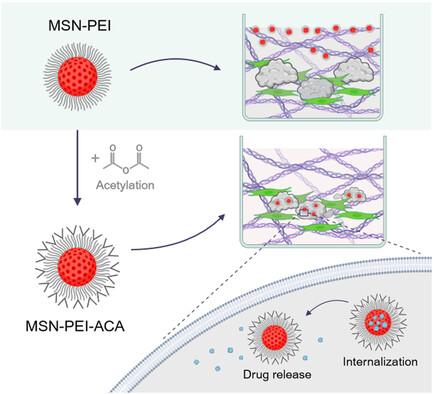
Surface Modification of Mesoporous Silica Nanoparticles as a Means to Introduce Inherent Cancer-Targeting Ability in a 3D Tumor Microenvironment
Mesoporous silica nanoparticles (MSNs) have emerged as promising drug carriers that can facilitate targeted anticancer drug delivery, but efficiency studies relying on active targeting mechanisms remain elusive. This study implements in vitro 3D cocultures, so-called microtissues, to model a physiologically relevant tumor microenvironment (TME) to examine the impact of surface-modified MSNs without targeting ligands on the internalization, cargo delivery, and cargo release in tumor cells and cancer-associated fibroblasts. Among these, acetylated MSNs most effectively localized in tumor cells in a 3D setting containing collagen, while other MSNs did so to a lesser degree, most likely due to remaining trapped in the extracellular matrix of the TME. Confocal imaging of hydrophobic model drug-loaded MSNs demonstrated effective cargo release predominantly in tumor cells, both in 2D and 3D cocultures. MSN-mediated delivery of an anticancer drug in the microtissues exhibited a significant reduction in tumor organoid size and enhanced the tumor-specific cytotoxic effects of a γ-secretase inhibitor, compared to the highly hydrophobic drug in free form. This inherent targeting potential suggests reduced off-target effects and increased drug efficacy, showcasing the promise of surface modification of MSNs as a means of direct cell-specific targeting and delivery for precise and successful targeted drug delivery.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
14.00
自引率
2.40%
发文量
0
期刊介绍:
Small Science is a premium multidisciplinary open access journal dedicated to publishing impactful research from all areas of nanoscience and nanotechnology. It features interdisciplinary original research and focused review articles on relevant topics. The journal covers design, characterization, mechanism, technology, and application of micro-/nanoscale structures and systems in various fields including physics, chemistry, materials science, engineering, environmental science, life science, biology, and medicine. It welcomes innovative interdisciplinary research and its readership includes professionals from academia and industry in fields such as chemistry, physics, materials science, biology, engineering, and environmental and analytical science. Small Science is indexed and abstracted in CAS, DOAJ, Clarivate Analytics, ProQuest Central, Publicly Available Content Database, Science Database, SCOPUS, and Web of Science.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


