SARS-CoV-2 在人类气道上皮细胞中诱发的启动细胞死亡事件。
IF 17.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
病毒诱导的细胞死亡是 COVID-19 病理学的一个关键因素。严重急性呼吸系统综合征冠状病毒 2(SARS-CoV-2)诱导的细胞死亡在骨髓细胞中的研究较多,但在其主要宿主细胞类型--表达血管紧张素转换酶 2(ACE2)的人气道上皮细胞(HAE)中的研究较少。SARS-CoV-2 可诱导 HAE 器官型培养物中的细胞凋亡、坏死和热凋亡。单细胞和稀释分析显示,坏死是感染细胞的主要细胞死亡事件,而未感染的旁观者则会发生凋亡,热凋亡发生在感染的后期。从机理上讲,在 HAE 和 COVID-19 患者的肺组织中,病毒 Z-RNA 与 Z-DNA 结合蛋白 1 (ZBP1) 结合会诱导坏死。在人类中,Delta(B.1.617.2)变异体比 Omicron(B1.1.529)变异体引起的疾病更严重,在动物模型中,Delta 与 Z-RNA/ZBP1 的相互作用、坏死和疾病严重程度呈数量级增长。因此,Delta 能诱导强大的 ZBP1 介导的坏死和更严重的疾病。本文章由计算机程序翻译,如有差异,请以英文原文为准。
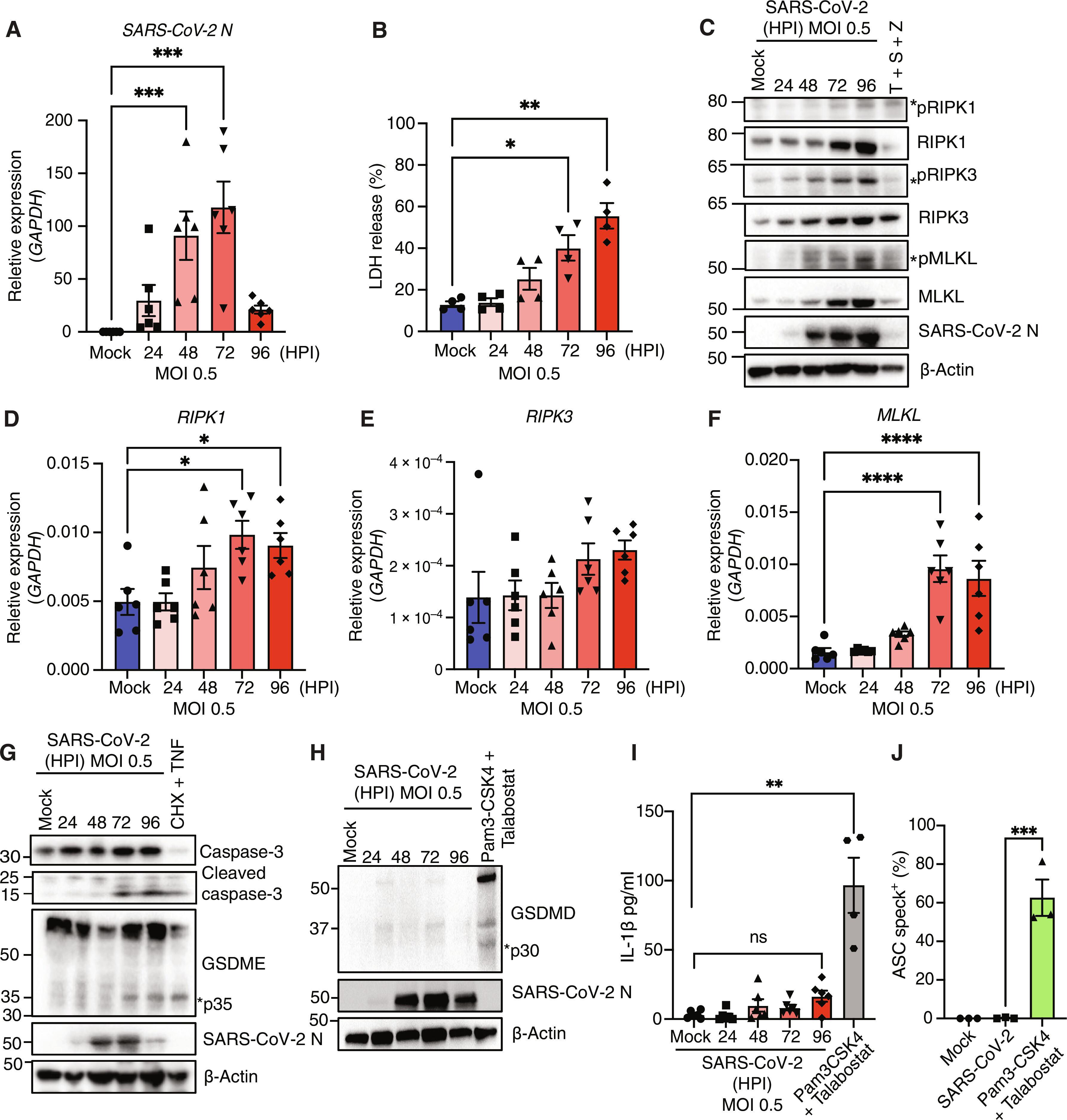
Initiator cell death event induced by SARS-CoV-2 in the human airway epithelium
Virus-induced cell death is a key contributor to COVID-19 pathology. Cell death induced by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is well studied in myeloid cells but less in its primary host cell type, angiotensin-converting enzyme 2 (ACE2)–expressing human airway epithelia (HAE). SARS-CoV-2 induces apoptosis, necroptosis, and pyroptosis in HAE organotypic cultures. Single-cell and limiting-dilution analysis revealed that necroptosis is the primary cell death event in infected cells, whereas uninfected bystanders undergo apoptosis, and pyroptosis occurs later during infection. Mechanistically, necroptosis is induced by viral Z-RNA binding to Z-DNA–binding protein 1 (ZBP1) in HAE and lung tissues from patients with COVID-19. The Delta (B.1.617.2) variant, which causes more severe disease than Omicron (B1.1.529) in humans, is associated with orders of magnitude–greater Z-RNA/ZBP1 interactions, necroptosis, and disease severity in animal models. Thus, Delta induces robust ZBP1-mediated necroptosis and more disease severity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Immunology
Immunology and Microbiology-Immunology
CiteScore
32.90
自引率
2.00%
发文量
183
期刊介绍:
Science Immunology is a peer-reviewed journal that publishes original research articles in the field of immunology. The journal encourages the submission of research findings from all areas of immunology, including studies on innate and adaptive immunity, immune cell development and differentiation, immunogenomics, systems immunology, structural immunology, antigen presentation, immunometabolism, and mucosal immunology. Additionally, the journal covers research on immune contributions to health and disease, such as host defense, inflammation, cancer immunology, autoimmunity, allergy, transplantation, and immunodeficiency. Science Immunology maintains the same high-quality standard as other journals in the Science family and aims to facilitate understanding of the immune system by showcasing innovative advances in immunology research from all organisms and model systems, including humans.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


