利用阈值光电子能谱研究丙炔自由基气相重组产生的 C6H6 产物的异构体分布。
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
丙炔基(C3H3)的共振稳定性使其成为极端环境中最重要的反应中间体之一,并赋予其足够长的寿命,使其能够在地球燃烧介质和太空冷分子云中重新结合。这使得丙炔自反应成为形成苯(第一个芳香环)的关键步骤,最终在各种环境中形成多环芳烃。在这项研究中,我们通过在丙炔与 F 原子反应的流动管中产生丙炔自由基,并通过质量选择阈值光电子能谱(TPES)探测反应产物,总共确定了包括苯在内的八种 C6H6 产物。本研究首次全面测量了丙炔自反应产物(4 毫巴、298 K 条件下)中已识别的八种 C6H6 异构体的支化率,此外还强调了使用异构体选择性 TPES 识别和量化反应产物的优缺点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
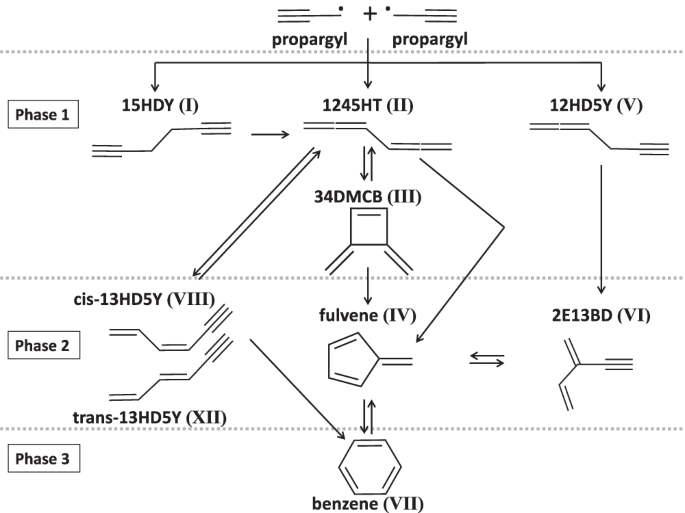
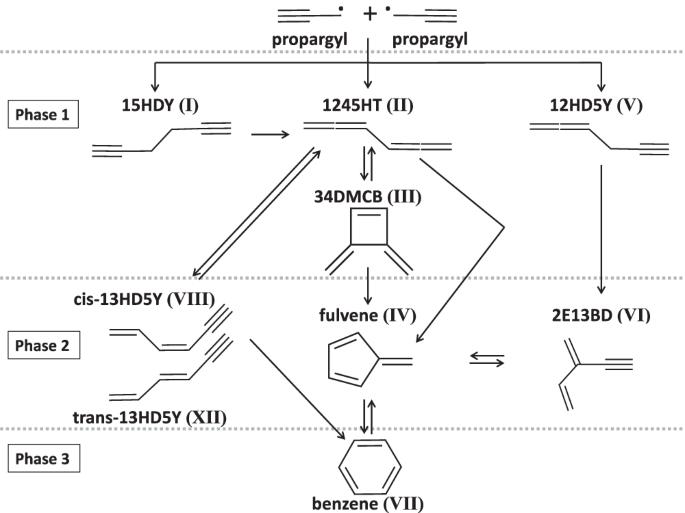
The isomer distribution of C6H6 products from the propargyl radical gas-phase recombination investigated by threshold-photoelectron spectroscopy
The resonance-stabilization of the propargyl radical (C3H3) makes it among the most important reactive intermediates in extreme environments and grants it a long enough lifetime to recombine in both terrestrial combustion media and cold molecular clouds in space. This makes the propargyl self-reaction a pivotal step in the formation of benzene, the first aromatic ring, to eventually lead to polycyclic aromatic hydrocarbons in a variety of environments. In this work, by producing propargyl radicals in a flow tube where propyne reacted with F atoms and probing the reaction products by mass-selected threshold-photoelectron spectroscopy (TPES), we identified eight C6H6 products in total, including benzene. On top of providing the first comprehensive measurements of the branching ratios of the eight identified C6H6 isomers in the propargyl self reaction products (4 mbar, 298 K conditions), this study also highlights the advantages and disadvantages of using isomer-selective TPES to identify and quantify reaction products. The propargyl radical (C3H3) self-reaction is a pivotal step in the formation of benzene in nature, but experimental validation for the complex reaction channels and products is challenging to obtain. Here, the authors produce propargyl radicals in a flow tube and report the branching ratios of eight identified C6H6 isomers in the propargyl self-reaction using isomer-selective threshold-photoelectron spectroscopy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


