特定 CD4+ T 细胞表型与 "抵抗 "结核分枝杆菌感染者的细菌控制有关
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
暴露于结核分枝杆菌(Mtb)的一部分人被我们称为 "抵抗者"(RSTR),尽管他们的临床检测结果连续呈阴性,但有证据表明他们的T细胞对Mtb特异性抗原产生了IFN-γ反应。在这里,我们发现 RSTR 中的 Mtb 特异性 T 细胞呈克隆性扩增,这证实了暴露于 Mtb 后适应性免疫反应的启动。与来自潜伏Mtb感染者的Mtb特异性T细胞相比,RSTR CD4+T细胞显示出TH17和调节性T细胞样功能程序的富集。利用公开数据集,我们发现这些 TH17 细胞样功能程序与南非潜伏 Mtb 感染青少年未发展为活动性结核病有关,也与非人灵长类动物的细菌控制有关。我们的研究结果表明,RSTR 可在暴露和免疫启动后成功控制 Mtb,并建立了一套 T 细胞生物标志物,以促进对这种临床表型的进一步研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
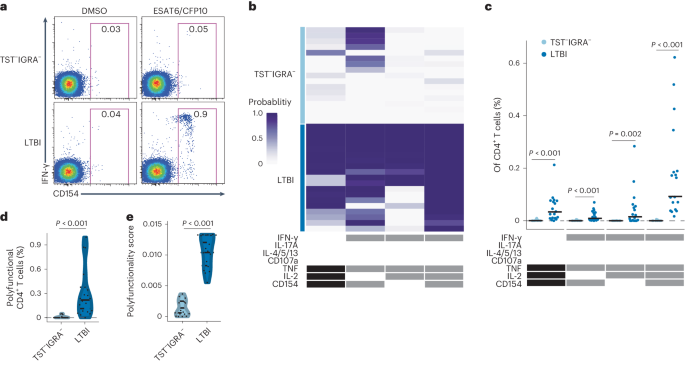
Specific CD4+ T cell phenotypes associate with bacterial control in people who ‘resist’ infection with Mycobacterium tuberculosis
A subset of individuals exposed to Mycobacterium tuberculosis (Mtb) that we refer to as ‘resisters’ (RSTR) show evidence of IFN-γ− T cell responses to Mtb-specific antigens despite serially negative results on clinical testing. Here we found that Mtb-specific T cells in RSTR were clonally expanded, confirming the priming of adaptive immune responses following Mtb exposure. RSTR CD4+ T cells showed enrichment of TH17 and regulatory T cell-like functional programs compared to Mtb-specific T cells from individuals with latent Mtb infection. Using public datasets, we showed that these TH17 cell-like functional programs were associated with lack of progression to active tuberculosis among South African adolescents with latent Mtb infection and with bacterial control in nonhuman primates. Our findings suggested that RSTR may successfully control Mtb following exposure and immune priming and established a set of T cell biomarkers to facilitate further study of this clinical phenotype. Seshadri, Davis and colleagues show that individuals who do not develop an infection with Mycobacterium tuberculosis (Mtb), despite exposure to the bacteria and expansion of CD4+ T cell clones specific to Mtb antigens, show enrichment of TH17 cell and T regulatory functional programs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


