溶酶体的压力感应可控制巨噬细胞中的 TFEB 反应
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
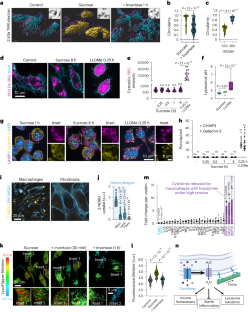
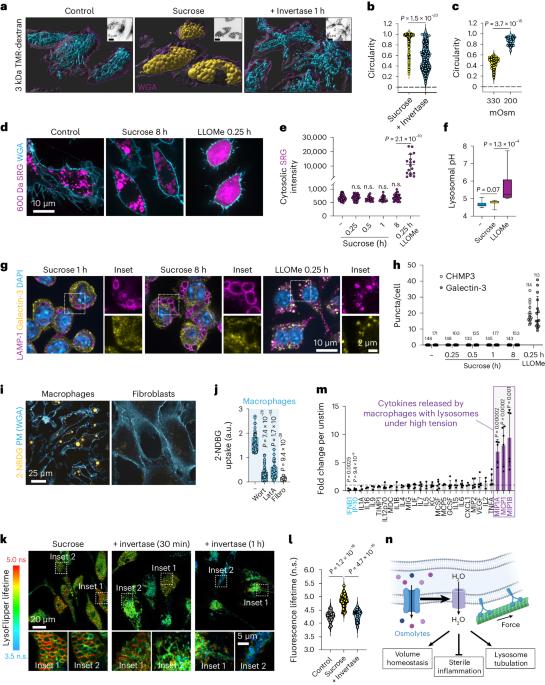
聚合物被内吞并被溶酶体酶水解,生成可运输的溶质。虽然对各种有机溶质通过质膜的运输进行了深入研究,但与之相比,对它们从内吞液持续流出到细胞质的必要性却知之甚少。采用特殊类型内吞作用(即吞噬和大蛋白内吞作用)的髓样细胞高度依赖这种转运途径来防止静水压的积累,否则就会抵消溶酶体的活力,包括囊泡化、管化和裂解。我们发现,溶酶体在不发生破裂的情况下,由于溶质外流缺陷而产生的这种压力无法保留管腔内的 Na+,从而使其与细胞质之间的梯度崩溃。这种阳离子 "泄漏 "是由常驻溶酶体的压力敏感通道介导的,并导致抑制 mTORC1,而 mTORC1 通常是由 Na+ 梯度驱动的 Na+ 偶联氨基酸转运体激活的。因此,转录因子 TFEB/TFE3 在溶酶体膨胀的巨噬细胞中变得活跃。除了在溶酶体生物生成过程中发挥作用外,TFEB/TFE3 的激活还会导致 MCP-1/CCL2 的释放。在受到代谢压力的组织中,内吞途径溶质外流缺陷会导致单核细胞招募增加。在这里,我们提出巨噬细胞对溶酶体上的压力传感途径做出反应,以协调溶酶体的生物生成以及髓系细胞的招募。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Pressure sensing of lysosomes enables control of TFEB responses in macrophages
Polymers are endocytosed and hydrolysed by lysosomal enzymes to generate transportable solutes. While the transport of diverse organic solutes across the plasma membrane is well studied, their necessary ongoing efflux from the endocytic fluid into the cytosol is poorly appreciated by comparison. Myeloid cells that employ specialized types of endocytosis, that is, phagocytosis and macropinocytosis, are highly dependent on such transport pathways to prevent the build-up of hydrostatic pressure that otherwise offsets lysosomal dynamics including vesiculation, tubulation and fission. Without undergoing rupture, we found that lysosomes incurring this pressure owing to defects in solute efflux, are unable to retain luminal Na+, which collapses its gradient with the cytosol. This cation ‘leak’ is mediated by pressure-sensitive channels resident to lysosomes and leads to the inhibition of mTORC1, which is normally activated by Na+-coupled amino acid transporters driven by the Na+ gradient. As a consequence, the transcription factors TFEB/TFE3 are made active in macrophages with distended lysosomes. In addition to their role in lysosomal biogenesis, TFEB/TFE3 activation causes the release of MCP-1/CCL2. In catabolically stressed tissues, defects in efflux of solutes from the endocytic pathway leads to increased monocyte recruitment. Here we propose that macrophages respond to a pressure-sensing pathway on lysosomes to orchestrate lysosomal biogenesis as well as myeloid cell recruitment. Cai et al. show that, in lysosomes under hydrostatic pressure in macrophages, lysosomal mechanosensitive channels cause a cation leak. This leads to the inhibition of mTORC1, activation of TFEB/TFE3 and release of monocyte chemoattractant proteins.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


