通过硼基添加剂驱动的自优化界面实现富锰基锂离子阴极的卓越速率性能
IF 9.1
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
富锂锰基正极材料(LRM)是一种很有前途的高能量密度锂电池正极材料,但在传统的六氟磷酸锂(LiPF6)基碳酸盐电解质中存在严重的副反应,导致界面不稳定和速率性能低下。本文提出了一种硼基添加剂驱动的自优化界面策略,在正极电解质外界面溶解低离子电导率的 LiF 纳米粒子,从而优化了界面成分,并提高了电解质中的锂离子迁移率。由于这些优点,LRM||锂电池在 200 次循环后,1C 下的容量保持率高达 92.19%,电压衰减低至 1.08 mV/次。这项研究为合理选择具有界面自优化特性的功能添加剂,以实现具有卓越速率性能的长寿命 LRM 提供了一个新的视角。本文章由计算机程序翻译,如有差异,请以英文原文为准。
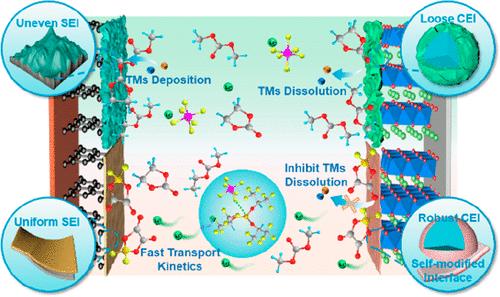
Exceptional Rate Performances of Li-Rich Mn-Based Cathodes Enabled by Boron-Based Additives-Driven Self-Optimized Interface
Li-rich Mn-based cathode material (LRM), as a promising cathode for high energy density lithium batteries, suffers from severe side reactions in conventional lithium hexafluorophosphate (LiPF6)-based carbonate electrolytes, leading to unstable interfaces and poor rate performances. Herein, a boron-based additives-driven self-optimized interface strategy is presented to dissolve low ionic conductivity LiF nanoparticles at the outer cathode electrolyte interface, leading to the optimized interfacial components, as well as the enhanced Li ion migration rate in electrolytes. Being attributed to these superiorities, the LRM||Li battery delivers a high-capacity retention of 92.19% at 1C after 200 cycles and a low voltage decay of 1.08 mV/cycle. This work provides a new perspective on the rational selection of functional additives with an interfacial self-optimized characteristic to achieve a long lifespan LRM with exceptional rate performances.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nano Letters
工程技术-材料科学:综合
CiteScore
16.80
自引率
2.80%
发文量
1182
审稿时长
1.4 months
期刊介绍:
Nano Letters serves as a dynamic platform for promptly disseminating original results in fundamental, applied, and emerging research across all facets of nanoscience and nanotechnology. A pivotal criterion for inclusion within Nano Letters is the convergence of at least two different areas or disciplines, ensuring a rich interdisciplinary scope. The journal is dedicated to fostering exploration in diverse areas, including:
- Experimental and theoretical findings on physical, chemical, and biological phenomena at the nanoscale
- Synthesis, characterization, and processing of organic, inorganic, polymer, and hybrid nanomaterials through physical, chemical, and biological methodologies
- Modeling and simulation of synthetic, assembly, and interaction processes
- Realization of integrated nanostructures and nano-engineered devices exhibiting advanced performance
- Applications of nanoscale materials in living and environmental systems
Nano Letters is committed to advancing and showcasing groundbreaking research that intersects various domains, fostering innovation and collaboration in the ever-evolving field of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


