动态溴空位介导的光催化三步三电子氧还原羟基自由基
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
羟基自由基(-OH)具有很强的氧化能力,在光催化领域受到广泛关注。然而,作为一种广泛应用于环境光催化领域的二维层状材料,BiOBr 无法催化产生 -OH,严重阻碍了其对有机污染物的高效降解。在本文中,我们提出了一种通过扩大晶面间距来生成 -OH 的有效方法。通过扩大 BiOBr 的 {001} 晶面间距,我们合成了超薄的 BiOBr-3 纳米片,Bi-Br 键的键能降低,有利于 Br- 的沉淀和溴空位(BrV)在光催化条件下的形成,从而促进分子氧的高效活化生成 -OH。通过原位红外光谱和自由基探针实验,阐明了光催化通过三个步骤和三个电子将分子氧还原为羟基自由基的机理。密度泛函理论计算表明,含有溴空位的 BiOBr-3 显著降低了 *OOH 的自由能垒,促进了 H2O2 的形成和还原成 -OH。与不含溴空位的 BiOBr 相比,含溴空位的 BiOBr-3 在降解三嗪类有机污染物方面表现出更高的光活性。通过使用毒性估计软件工具和基于结构-活性关系的定量方法以及 HepG2 细胞活力检测,阿特拉津降解溶液的毒性显著降低。本文章由计算机程序翻译,如有差异,请以英文原文为准。
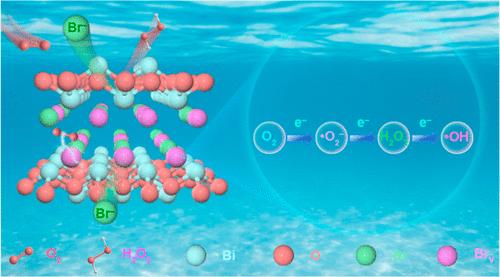
Dynamic Bromine Vacancy-Mediated Photocatalytic Three-Step Three-Electron Oxygen Reduction to Hydroxyl Radicals
Hydroxyl radicals (•OH), recognized for their strong oxidizing ability, have garnered extensive attention in the field of photocatalysis. However, as a two-dimensional layered material widely employed in the field of environmental photocatalysis, BiOBr is incapable of catalyzing the generation of •OH, severely impeding its efficient degradation of organic pollutants. In this paper, we propose an efficient approach to generate •OH by expanding the spacing between crystal faces. Through the expansion of the {001} crystal face spacing of BiOBr, we synthesized ultrathin BiOBr-3 nanosheets with reduced bond energy of the Bi–Br bond, which favored the precipitation of Br– and the formation of bromine vacancies (BrV) under photocatalytic conditions, thereby promoting the efficient activation of molecular oxygen to generate •OH. The mechanism of photocatalytic reduction of molecular oxygen to hydroxyl radicals by three steps and three electrons was elucidated by in situ infrared spectroscopy and free radical probe experiments. Density functional theory calculations indicated that BiOBr-3 containing bromine vacancies significantly reduced the free energy barrier of *OOH, facilitating the formation of H2O2 and the reduction to •OH. In comparison to BiOBr without BrV, BiOBr-3 containing BrV demonstrated higher photoactivity toward degradation of triazine organic pollutants. The toxicity of the atrazine degradation solution was significantly reduced through the use of the toxicity estimation software tool and quantitative structure–activity relationship-based methods, as well as HepG2 cell viability detection.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


