RNA 通过与 GM130 形成缩聚体来支撑高尔基体带
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
哺乳动物的高尔基体由横向连接成连续带状结构的堆栈组成。在应激条件下,带状结构的完整性和功能会被破坏,但其分子机制仍不清楚。在这里,我们发现带状结构是由 RNA 和高尔基体基质蛋白 GM130(GOLGA2)的生物分子凝聚物维持的。我们发现 GM130 是一种膜结合 RNA 结合蛋白,可直接将 RNA 和相关 RNA 结合蛋白招募到高尔基体膜上。细胞中 RNA 或 GM130 的急性降解会破坏带状结构。在应激条件下,RNA 与 GM130 分离,带状结构脱节,但细胞从应激中恢复后,带状结构又会恢复。当 GM130 在细胞中过度表达时,会形成依赖于 RNA 的液态凝结物。GM130 的氨基末端含有一个固有紊乱结构域,该结构域与 RNA 结合,诱导液-液相分离。这些共凝结物足以连接纯化的高尔基体膜,将横向连接的膜堆重建为带状结构。这些研究共同表明,RNA 是一种结构性生物聚合物,它与 GM130 一起维持着高尔基体带状结构的完整性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
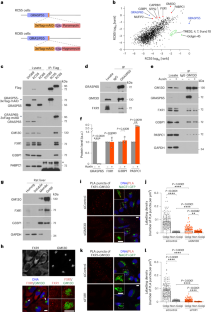
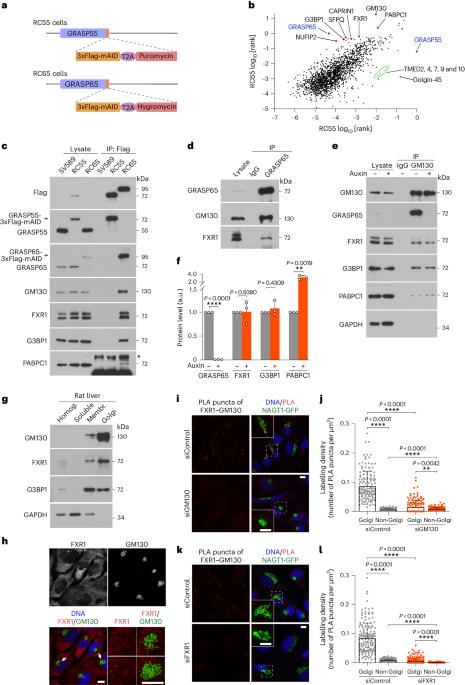
RNA scaffolds the Golgi ribbon by forming condensates with GM130
The mammalian Golgi is composed of stacks that are laterally connected into a continuous ribbon-like structure. The integrity and function of the ribbon is disrupted under stress conditions, but the molecular mechanisms remain unclear. Here we show that the ribbon is maintained by biomolecular condensates of RNA and the Golgi matrix protein GM130 (GOLGA2). We identify GM130 as a membrane-bound RNA-binding protein, which directly recruits RNA and associated RNA-binding proteins to the Golgi membrane. Acute degradation of RNA or GM130 in cells disrupts the ribbon. Under stress conditions, RNA dissociates from GM130 and the ribbon is disjointed, but after the cells recover from stress the ribbon is restored. When overexpressed in cells, GM130 forms RNA-dependent liquid-like condensates. GM130 contains an intrinsically disordered domain at its amino terminus, which binds RNA to induce liquid–liquid phase separation. These co-condensates are sufficient to link purified Golgi membranes, reconstructing lateral linking of stacks into a ribbon-like structure. Together, these studies show that RNA acts as a structural biopolymer that together with GM130 maintains the integrity of the Golgi ribbon. Zhang and Seemann show that GM130 forms a complex with RNA-binding proteins. RNA binding of GM130 induces liquid–liquid phase separation and these co-condensates function to link the Golgi ribbon.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


