胸腺先天性类 T 细胞直接呈现炎症相关的自身抗原,诱导自身反应性 CD8+胸腺细胞的清除
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
构成炎症反应成分的各种自抗原的上调与病原体衍生的外来抗原的出现在空间和时间上重叠。因此,区分这些炎症相关自抗原和病原体衍生分子是适应性免疫系统面临的一项独特挑战。在这里,我们证明了 CD8+ T 细胞对 T 细胞衍生的炎症相关自身抗原的耐受性是在胸腺中有效诱导的,并得到了表达这些分子的细胞类型冗余的支持。除胸腺上皮细胞外,还包括胸腺嗜酸性粒细胞和先天性类 T 细胞,这些细胞表达所有主要活化 T 细胞亚群的特征分子。我们的研究表明,微量的先天性类 T 细胞进行 T 细胞对 T 细胞的直接抗原呈递足以消除自反应性 CD8+ 胸腺细胞。对这种效应分子的耐受性至关重要,因为胸腺中与炎症相关的单一自身抗原的减少会破坏这种耐受性,从而导致整个效应T细胞亚群的自身免疫性消除。本文章由计算机程序翻译,如有差异,请以英文原文为准。
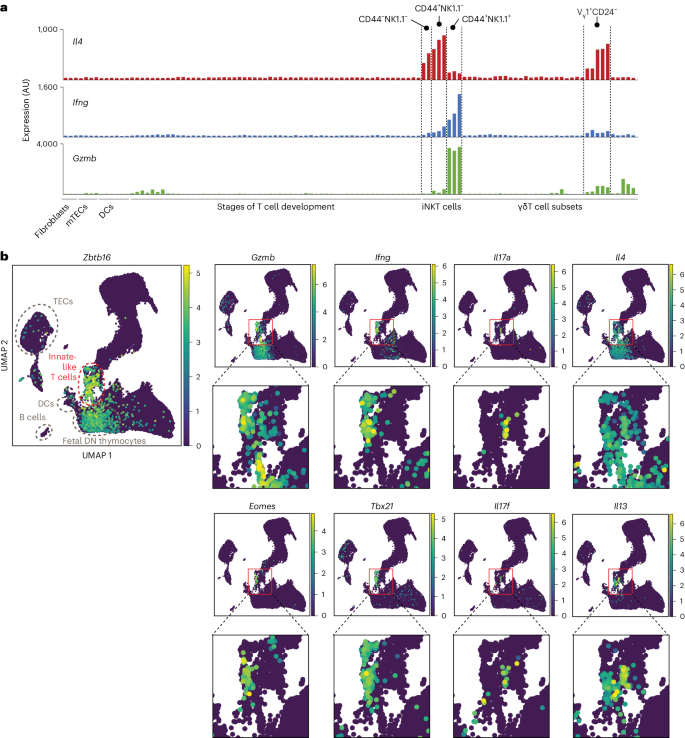
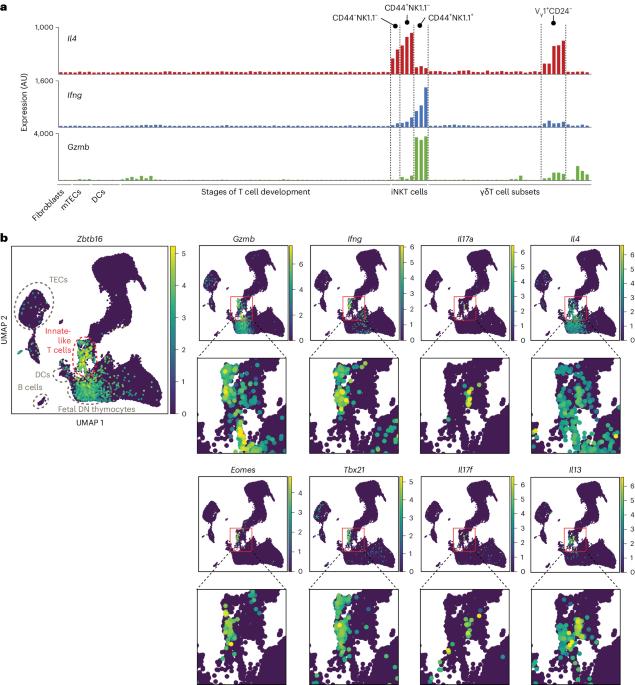
Direct presentation of inflammation-associated self-antigens by thymic innate-like T cells induces elimination of autoreactive CD8+ thymocytes
Upregulation of diverse self-antigens that constitute components of the inflammatory response overlaps spatially and temporally with the emergence of pathogen-derived foreign antigens. Therefore, discrimination between these inflammation-associated self-antigens and pathogen-derived molecules represents a unique challenge for the adaptive immune system. Here, we demonstrate that CD8+ T cell tolerance to T cell-derived inflammation-associated self-antigens is efficiently induced in the thymus and supported by redundancy in cell types expressing these molecules. In addition to thymic epithelial cells, this included thymic eosinophils and innate-like T cells, a population that expressed molecules characteristic for all major activated T cell subsets. We show that direct T cell-to-T cell antigen presentation by minute numbers of innate-like T cells was sufficient to eliminate autoreactive CD8+ thymocytes. Tolerance to such effector molecules was of critical importance, as its breach caused by decreased thymic abundance of a single model inflammation-associated self-antigen resulted in autoimmune elimination of an entire class of effector T cells. The thymus harbors a complex constitutively active inflammatory network with innate-like T cells representing one of its central nodes. Here, the authors show that these cells can induce tolerance to inflammation-associated self-antigens, a class of molecules that otherwise largely mirrors the spatial and temporal distribution of pathogen-derived antigens.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


