5-Arylpenta-2,4-dienoic Acids 的模板指导选择性光二聚化反应。
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们开发了一种高效的方法,可对 5-芳戊-2,4-二烯酸(即乙烯基肉桂酸)进行选择性光二聚化。使用 1,8-二羟基萘作为模板可确保两个反应烯烃的邻近性,因此照射模板结合的二烯酸可得到单[2 + 2]环加成产物,产率高(达 99%),为单一区域异构体,非对映选择性高(dr = 3:1 至 13:1)。化合物 12a、16a 和 22a 的几何和立体化学特征通过 X 射线晶体学进行了分析。本文章由计算机程序翻译,如有差异,请以英文原文为准。
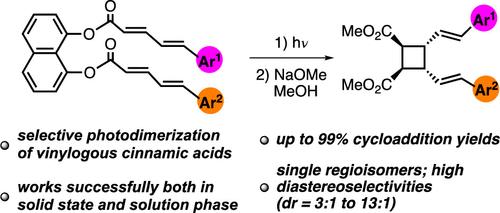
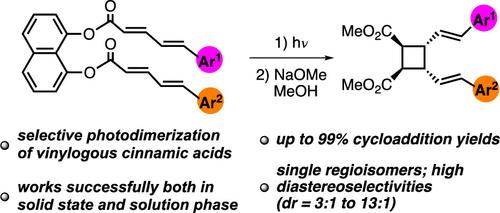
Template-Directed Selective Photodimerization Reactions of 5-Arylpenta-2,4-dienoic Acids
We developed an efficient method that enables selective photodimerization of 5-arylpenta-2,4-dienoic acids (i.e., vinylogous cinnamic acids). The use of 1,8-dihydroxynaphthalene as a template ensures proximity of the two reacting olefins so that irradiation of template-bound dienoic acids gives mono [2 + 2] cycloaddition products in good to excellent yields (up to 99%), as single regioisomers, and with high diastereoselectivities (dr = 3:1 to 13:1). The geometrical and stereochemical features of compounds 12a, 16a, and 22a were analyzed by X-ray crystallography.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


