以结核分枝杆菌特异性非必需膜蛋白 Rv2113 为靶标的抗结核药草素
IF 6.6
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
抗菌素耐药性的蔓延促使人们需要机制不同于现有抗生素的抗结核杆菌(Mtb)新药。此前,茜草素被认为是一种很有前景的抗结核药物,它代表了一类具有不寻常 (Z)-2,3 二氨基丙烯酰胺单元的疏水性环肽。在这里,我们研究了其抗结核特性的分子机制。通过结构-活性关系研究,我们确定了与抗菌活性相关的结构决定因素。Callyaerins是一种抑菌剂,对Mtb(包括广泛耐药菌株)具有选择性活性,对人体细胞的细胞毒性极小,而且具有良好的细胞内活性。通过结合突变体筛选和各种化学蛋白质组学方法,我们发现卡来霉素以非必需的、Mtb 特异性膜蛋白 Rv2113 为靶标,引发了复杂的蛋白质组失调,其特征是脂质生物合成、细胞分裂、DNA 修复和复制的全面下调。因此,我们的研究将 Rv2113 确定为以前未曾描述过的 Mtb 特异性药物靶点,并证明非必要蛋白也可能是抗霉菌药物的有效靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
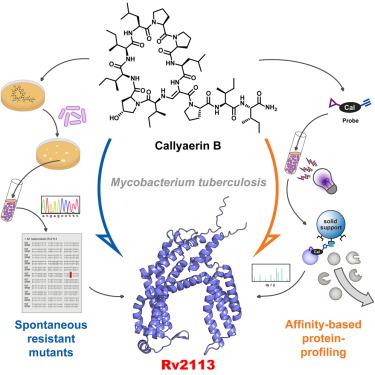
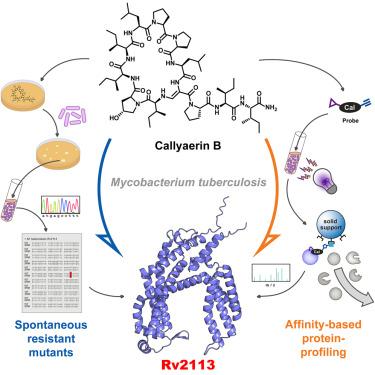
The anti-tubercular callyaerins target the Mycobacterium tuberculosis-specific non-essential membrane protein Rv2113
Spread of antimicrobial resistances urges a need for new drugs against Mycobacterium tuberculosis (Mtb) with mechanisms differing from current antibiotics. Previously, callyaerins were identified as promising anti-tubercular agents, representing a class of hydrophobic cyclopeptides with an unusual (Z)-2,3-di-aminoacrylamide unit. Here, we investigated the molecular mechanisms underlying their antimycobacterial properties. Structure-activity relationship studies enabled the identification of structural determinants relevant for antibacterial activity. Callyaerins are bacteriostatics selectively active against Mtb, including extensively drug-resistant strains, with minimal cytotoxicity against human cells and promising intracellular activity. By combining mutant screens and various chemical proteomics approaches, we showed that callyaerins target the non-essential, Mtb-specific membrane protein Rv2113, triggering a complex dysregulation of the proteome, characterized by global downregulation of lipid biosynthesis, cell division, DNA repair, and replication. Our study thus identifies Rv2113 as a previously undescribed Mtb-specific drug target and demonstrates that also non-essential proteins may represent efficacious targets for antimycobacterial drugs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


