免疫检查点抑制剂介导的心肌炎:心脏中的 CTLA4、PD1 和 LAG3
IF 72.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
免疫检查点抑制剂(ICIs)给肿瘤学带来了革命性的变化,近50%的癌症患者有资格接受ICIs治疗。然而,接受 ICI 治疗的患者面临着免疫相关毒性的风险,这些毒性可影响任何器官。ICI靶向细胞毒性T淋巴细胞相关抗原4(CTLA4)、程序性细胞死亡蛋白1(PD1)和PD1配体1(PDL1)导致的心肌炎是一种不常见但可能致命的并发症。ICI 介导的心肌炎(ICI-myocarditis)是一个日益增长的临床实体,这是因为 ICIs 的广泛使用、临床认知度的提高以及联合 ICI 治疗的使用日益增多,而联合 ICI 治疗是 ICI-myocarditis(ICI-myocarditis)的一个有据可查的危险因素。在本综述中,我们将从基础和机理的角度来探讨 ICI-心肌炎,综合临床前模型和患者样本的最新数据。我们认为,从机理上理解免疫检查点分子的基本生物学特性可能会对疾病过程产生新的认识,从而改进诊断和治疗方法。ICI-心肌炎综合征是一种新型疾病,我们对心脏免疫检查点的了解也刚刚起步。然而,对病理生理学的研究将有助于更好地对患者进行风险分层、改进诊断方法并为患者提供精准治疗。本文章由计算机程序翻译,如有差异,请以英文原文为准。
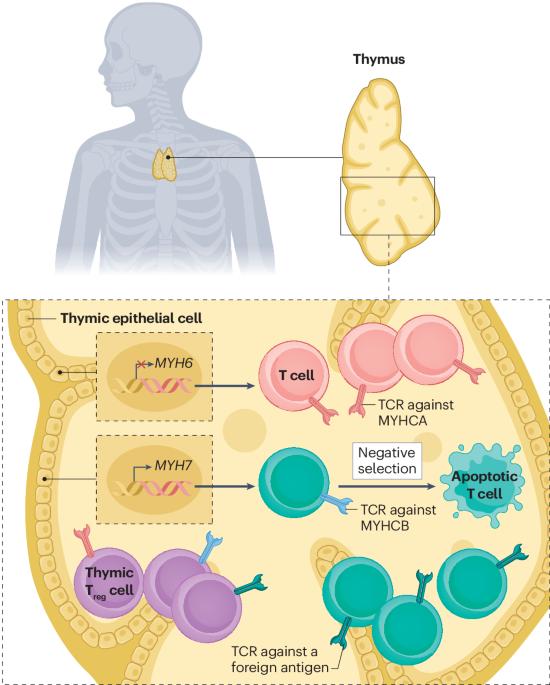
Immune-checkpoint inhibitor-mediated myocarditis: CTLA4, PD1 and LAG3 in the heart
Immune-checkpoint inhibitors (ICIs) have revolutionized oncology, with nearly 50% of all patients with cancer eligible for treatment with ICIs. However, patients on ICI therapy are at risk for immune-related toxicities that can affect any organ. Inflammation of the heart muscle, known as myocarditis, resulting from ICI targeting cytotoxic T lymphocyte-associated antigen 4 (CTLA4), programmed cell death protein 1 (PD1) and PD1 ligand 1 (PDL1) is an infrequent but potentially fatal complication. ICI-mediated myocarditis (ICI-myocarditis) is a growing clinical entity given the widespread use of ICIs, its increased clinical recognition and growing use of combination ICI treatment, a well-documented risk factor for ICI-myocarditis. In this Review, we approach ICI-myocarditis from a basic and mechanistic perspective, synthesizing the recent data from both preclinical models and patient samples. We posit that mechanistic understanding of the fundamental biology of immune-checkpoint molecules may yield new insights into disease processes, which will enable improvement in diagnostic and therapeutic approaches. The syndrome of ICI-myocarditis is novel, and our understanding of immune checkpoints in the heart is in its nascency. Yet, investigations into the pathophysiology will inform better patient risk stratification, improved diagnostics and precision-based therapies for patients. Despite the success of immune-checkpoint inhibitors, many patients are at risk of developing immune-related adverse events. One of these is myocarditis or inflammation of the heart. Munir, Gutierrez and colleagues describe the data from preclinical models and patient samples, which have begun to provide a mechanistic understanding of myocarditis resulting from immune-checkpoint inhibitors, and present suggestions for improving both the diagnosis and treatment of patients experiencing this immune-related toxicity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Cancer
医学-肿瘤学
CiteScore
111.90
自引率
0.40%
发文量
97
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Cancer, a part of the Nature Reviews portfolio of journals, aims to be the premier source of reviews and commentaries for the scientific communities it serves. The correct abbreviation for abstracting and indexing purposes is Nat. Rev. Cancer. The international standard serial numbers (ISSN) for Nature Reviews Cancer are 1474-175X (print) and 1474-1768 (online). Unlike other journals, Nature Reviews Cancer does not have an external editorial board. Instead, all editorial decisions are made by a team of full-time professional editors who are PhD-level scientists. The journal publishes Research Highlights, Comments, Reviews, and Perspectives relevant to cancer researchers, ensuring that the articles reach the widest possible audience due to their broad scope.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


