将 MAD2B 作为缺血性中风治疗的靶点。
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
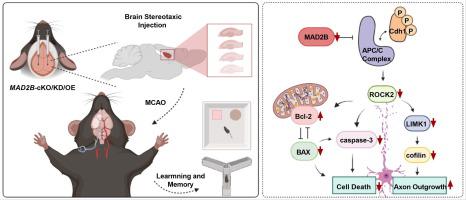
导言:中风后认知障碍是脑缺血致残的主要原因之一。MAD2B是Cdh1/APC的抑制剂,成熟神经元中Cdh1/APC功能的丧失会增加ROCK2的活性,导致突触可塑性的改变和小鼠神经元记忆力的丧失。MAD2B是否通过ROCK2调节脑缺血时的学习记忆能力尚不清楚:目的:我们研究了 MAD2B 在脑缺血诱导的认知功能障碍中的作用和机制:方法:用免疫印迹法检测MAD2B及其下游相关分子的表达,并在大脑中动脉闭塞(MCAO)和氧-葡萄糖剥夺/再氧合(OGD/R)后用神经保护剂干预。我们构建了MAD2B-cKO特异性基因敲除小鼠,通过慢病毒注射在小鼠海马中敲除并过表达MAD2B,利用MCAO模拟脑缺血,并通过Y-迷宫和新物体识别测试等动物行为探讨MAD2B在卒中后认知障碍(PSCI)中的作用。然后检测了MAD2B/ROCK2、下游分子和凋亡相关分子的表达。最后,使用ROCK2抑制剂和shRNA-ROCK2慢病毒干预ROCK2的表达:结果:MCAO和OGD/R后,MAD2B及其下游分子的表达增加。结果:MAD2B 及其下游分子的表达在 MCAO 和 OGD/R 后增加,但在使用神经保护剂治疗后下降。在海马中删除 MAD2B 可改善 MCAO 小鼠的记忆和学习障碍,并提高运动协调能力。相反,在海马中过表达 MAD2B 则会加重学习和记忆障碍。删除 MAD2B 会导致 ROCK2/LIMK1/cofilin 的下调。它能有效降低缺血诱导的 BAX 和裂解的 caspase-3 的上调,而 MAD2B 的过表达能逆转这种上调。抑制或敲除原代培养神经元中 ROCK2 的表达可导致 LIMK1/cofilin 表达下调,并减少缺血诱导的细胞凋亡相关分子的表达:我们的研究结果表明,MAD2B通过Rock2影响神经元凋亡,从而影响神经功能和脑梗塞。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Targeting MAD2B as a strategy for ischemic stroke therapy
Introduction
Post-stroke cognitive impairment is one of the major causes of disability due to cerebral ischemia. MAD2B is an inhibitor of Cdh1/APC, and loss of Cdh1/APC function in mature neurons increases ROCK2 activity, leading to changes in synaptic plasticity and memory loss in mouse neurons. Whether MAD2B regulates learning memory capacity through ROCK2 in cerebral ischemia is not known.
Objectives
We investigated the role and mechanism of MAD2B in cerebral ischemia-induced cognitive dysfunction.
Methods
The expression of MAD2B and its downstream related molecules was detected by immunoblotting and intervened with neuroprotectants after middle cerebral artery occlusion (MCAO) and oxygen-glucose deprivation/reoxygenation (OGD/R). We constructed MAD2B-cKO-specific knockout mice, knocked down and overexpressed MAD2B in mouse hippocampus by lentiviral injection in brain stereotaxis, modeled cerebral ischemia by using MCAO, and explored the role of MAD2B in post-stroke cognitive impairment (PSCI) by animal behaviors such as Y-maze and Novel object recognition test. Then the expression of MAD2B/ROCK2, downstream molecules and apoptosis-related molecules was detected. Finally, ROCK2 expression was intervened using its inhibitor and shRNA-ROCK2 lentivirus.
Results
The expression of MAD2B and its downstream molecules increased after MCAO and OGD/R. Nonetheless, this expression underwent a decline post-therapy with neuroprotective agents. Deletion of MAD2B in the hippocampus ameliorated memory and learning deficits and improved motor coordination in MCAO mice. Conversely, the overexpression of MAD2B in the hippocampus exacerbated learning and memory deficits. Deletion of MAD2B resulted in the downregulation of ROCK2/LIMK1/cofilin. It effectively reduced ischemia-induced upregulation of BAX and cleaved caspase-3, which could be reversed by MAD2B overexpression. Inhibition or knockdown of ROCK2 expression in primary cultured neurons led to the downregulation of LIMK1/cofilin expression and reduced the expression of apoptosis-associated molecules induced by ischemia.
Conclusions
Our findings suggest that MAD2B affects neuronal apoptosis via Rock2, which affects neurological function and cerebral infarction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


