设计用于 HIV-1 蛋白酶抑制剂的取代四氢呋喃衍生物:合成、生物学评价和 X 射线结构研究。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们设计并合成了取代的四氢呋喃衍生物,作为一系列强效 HIV-1 蛋白酶抑制剂的 P2 配体。以脂肪酶-PS 催化的酶解为关键步骤,立体选择性地合成了具有光学活性的四氢呋喃衍生物的两种对映体。这些四氢呋喃衍生物旨在促进与 HIV-1 蛋白酶活性位点 S2 亚位的骨架原子之间的氢键和范德华相互作用。几种抑制剂显示出非常强的 HIV-1 蛋白酶抑制活性。抑制剂结合 HIV-1 蛋白酶的高分辨率 X 射线晶体结构为了解配体结合位点在活性位点中的相互作用提供了重要信息。本文章由计算机程序翻译,如有差异,请以英文原文为准。
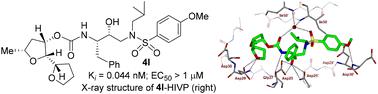
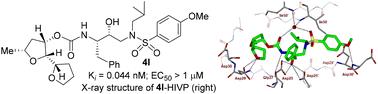
Design of substituted tetrahydrofuran derivatives for HIV-1 protease inhibitors: synthesis, biological evaluation, and X-ray structural studies†‡
Substituted tetrahydrofuran derivatives were designed and synthesized to serve as the P2 ligand for a series of potent HIV-1 protease inhibitors. Both enantiomers of the tetrahydrofuran derivatives were synthesized stereoselectivity in optically active forms using lipase-PS catalyzed enzymatic resolution as the key step. These tetrahydrofuran derivatives are designed to promote hydrogen bonding and van der Waals interactions with the backbone atoms in the S2 subsite of the HIV-1 protease active site. Several inhibitors displayed very potent HIV-1 protease inhibitory activity. A high-resolution X-ray crystal structure of an inhibitor-bound HIV-1 protease provided important insight into the ligand binding site interactions in the active site.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


