吸入β-葡聚糖-壳聚糖-聚(乳酸共聚乙醇)酸纳米颗粒可提高肺泡巨噬细胞中利福平的浓度,用于治疗结核病
IF 3.7
4区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
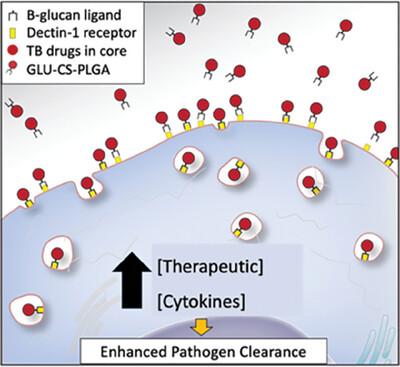
尽管结核病(TB)有多种治疗方法,但每年新增病例≈1,000 万,死亡人数达 150 万,因此需要新的治疗方法。临床治疗的主要问题包括治疗时间过长,这与患者的不依从性有关;以及细胞药物渗透性差,导致耐药菌株的产生。这项研究强调了β-葡聚糖-壳聚糖(CS)聚(乳酸共聚乙醇)酸(PLGA)纳米粒子作为肺结核治疗的免疫刺激辅助药物的潜力。为了便于向肺泡巨噬细胞给药,我们开发了一种 CS-PLGA 纳米粒子,其核心成分为利福平,表面配体为β-葡聚糖,以刺激免疫系统。小鼠经口咽吸入单剂量的纳米颗粒或游离利福平。药代动力学研究显示,利福平在体内具有持续释放特性,可持续一周以上。此外,综合分析表明,细胞因子图谱显示,利福平可刺激先天性免疫系统,同时,组织学检查和白蛋白肺渗漏评估显示,利福平对肺泡上皮细胞没有有害影响。这些发现共同为开发治疗结核病的新型辅助免疫疗法奠定了坚实的基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Inhalational Delivery of β‐glucan‐chitosan‐poly(lactic co‐glycolic) acid Nanoparticles Enhance Alveolar Macrophage Rifampin Concentrations for the Treatment of Tuberculosis
Despite multiple treatments for tuberculosis (TB), there are ≈10 million new cases and 1.5 million deaths annually, warranting the need for new therapeutics. Major clinical treatment issues include the length of treatment which is associated with patient non‐compliance; and poor cellular drug penetration leading to the generation of drug‐resistant strains. This study underscores the potential of β‐glucan‐chitosan (CS) poly(lactic co‐glycolic) acid (PLGA) nanoparticles as a promising immunostimulatory adjunct for TB treatment. To facilitate drug delivery to alveolar macrophage, a CS‐PLGA nanoparticle is developed containing rifampin in the core with β‐glucan as a surface ligand, to stimulate the immune system. Mice are administered a single dose of nanoparticles or free rifampin by oropharyngeal aspiration. Pharmacokinetic investigations reveal sustained release properties of rifampin in vivo, extending over a week. Furthermore, comprehensive analysis indicates stimulation of the innate immune system, as evidenced by cytokine profiling, while concurrently revealing no detrimental effects on the alveolar epithelium, as indicated by histological examination and albumin lung leak assessment. These findings collectively establish a strong foundation for the development of a novel adjuvant immunotherapy approach for TB.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Therapeutics
Pharmacology, Toxicology and Pharmaceutics-Pharmaceutical Science
CiteScore
7.10
自引率
2.20%
发文量
130
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


