扩展药代动力学利用辐射改善特定位点原药活化
IF 10.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
放疗是治疗癌症的常用方法,放疗沉积的局部能量具有化学解笼原药的潜力;然而,要证明原药活化在体内具有持续性并真正定位在肿瘤上而不影响靶外组织一直是个挑战。为了解决这个问题,我们开发了一系列新型苯基叠氮化物笼型辐射激活化疗药物结合物,并建立了一个计算框架来了解相应的药代动力学和药效学(PK/PD)行为。我们特别关注了一种与白蛋白结合的单甲基乌司他丁 E(MMAE)原药,发现它能阻断小鼠体内的肿瘤生长,向辐照肿瘤组织输送的活化药物量是未辐照组织的 130 倍,效率是未与白蛋白结合原药的 7.5 倍,而且与游离药物或可被酪蛋白酶活化的药物相比没有明显的毒性。这些数据为药物作用的计算建模提供了指导,建模结果表明,扩展的药代动力学可以改善药物的局部活化和累积活化,特别是对于血管渗透性和扩散性较低的有效载荷,尤其是对于连续数周每天接受常规放疗的患者。因此,这项研究提供了一个定量的 PK/PD 框架,并通过实验证明了延长原药的循环时间可以提高其在体内的局部活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
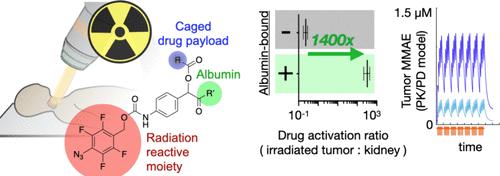
Extended Pharmacokinetics Improve Site-Specific Prodrug Activation Using Radiation
Radiotherapy is commonly used to treat cancer, and localized energy deposited by radiotherapy has the potential to chemically uncage prodrugs; however, it has been challenging to demonstrate prodrug activation that is both sustained in vivo and truly localized to tumors without affecting off-target tissues. To address this, we developed a series of novel phenyl-azide-caged, radiation-activated chemotherapy drug-conjugates alongside a computational framework for understanding corresponding pharmacokinetic and pharmacodynamic (PK/PD) behaviors. We especially focused on an albumin-bound prodrug of monomethyl auristatin E (MMAE) and found it blocked tumor growth in mice, delivered a 130-fold greater amount of activated drug to irradiated tumor versus unirradiated tissue, was 7.5-fold more efficient than a non albumin-bound prodrug, and showed no appreciable toxicity compared to free or cathepsin-activatable drugs. These data guided computational modeling of drug action, which indicated that extended pharmacokinetics can improve localized and cumulative drug activation, especially for payloads with low vascular permeability and diffusivity and particularly in patients receiving daily treatments of conventional radiotherapy for weeks. This work thus offers a quantitative PK/PD framework and proof-of-principle experimental demonstration of how extending prodrug circulation can improve its localized activity in vivo.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Central Science
Chemical Engineering-General Chemical Engineering
CiteScore
25.50
自引率
0.50%
发文量
194
审稿时长
10 weeks
期刊介绍:
ACS Central Science publishes significant primary reports on research in chemistry and allied fields where chemical approaches are pivotal. As the first fully open-access journal by the American Chemical Society, it covers compelling and important contributions to the broad chemistry and scientific community. "Central science," a term popularized nearly 40 years ago, emphasizes chemistry's central role in connecting physical and life sciences, and fundamental sciences with applied disciplines like medicine and engineering. The journal focuses on exceptional quality articles, addressing advances in fundamental chemistry and interdisciplinary research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


