通过离子催化剂控制的交叉偶联获得固有手性的间苯二酚空穴剂
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
空穴带已成为超分子化学中的重要结构。然而,通过大环边缘的异功能化实现结构多样性和可调性仍然是一项艰巨的挑战。举个简单的例子,将 C4v 对称支架逐步转化为固有手性 ABCD 模式在合成上并不可行,因为理论产率低(0.8%),而且需要进行色谱对映体分离。通过对离子手性钯催化剂进行工程化,可以在大边缘上安装包括芳基、烯基、炔基和氨基在内的多种官能团,并具有很高的位点选择性和立体选择性。根据偶联伙伴的编排,我们建立了一个可调整的步骤协议,以提供设计的 ABCD 型空穴剂。实验和计算研究揭示了离子催化剂与底物相互作用所产生的协同静电引导和静电催化作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
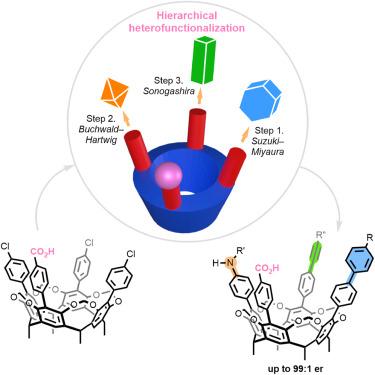
Inherently chiral resorcinarene cavitands through ionic catalyst-controlled cross-coupling
Cavitands have emerged as privileged architectures in supramolecular chemistry. Nonetheless, achieving structural diversity and tunability through heterofunctionalization along the rims of macrocycles has remained a formidable challenge. As a rudimental example, stepwise conversion of C4v-symmetric scaffolds to inherently chiral ABCD patterns is synthetically impractical owing to the low theoretical yields (0.8%) and the need for chromatographic enantioseparation.
Herein, we report a catalytic desymmetrization strategy to access inherently chiral cavitands. Through engineering ionic chiral palladium catalysts, diverse functionalities, including aryl, alkenyl, alkynyl, and amino groups, can be installed on the large rims with high site- and stereoselectivity. An adaptable stepwise protocol has been established to furnish designer ABCD-type cavitands in accordance with the choreography of coupling partners. Experimental and computational studies reveal synergistic electrostatic steering and electrostatic catalysis by the ionic catalyst–substrate interactions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


