通过光氧化和镍催化实现 α-氨基 C(sp3)-H 键的对映选择性烷基化
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
催化对映选择性地构建 C(sp3)-C(sp3)键仍然是有机合成中的一项重大挑战。一种特别有前景的方法是使用过渡金属催化 C(sp3)-H 功能化。然而,非酸性 C(sp3)-H键的对映选择性烷基化的一般策略尚未开发出来。在此,我们提出了一个统一的平台,利用光氧化和镍催化与广泛存在的氧化还原活性酯相结合,对α-氨基 C(sp3)-H 键进行对映选择性(三去甲)甲基化和烷基化。这种技术可激活两种偶联剂,形成以碳为中心的自由基,然后通过手性镍催化剂进行不对称偶联。这种策略的独特之处在于它能够分别控制自由基的生成和交叉偶联,便于在不对称催化中使用瞬时生成的烷基自由基(包括高活性甲基自由基),从而加快了对映体丰富的生物活性生物碱的合成,并为促进不对称 C(sp3)-C(sp3) 键的形成提供了一种前景广阔的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
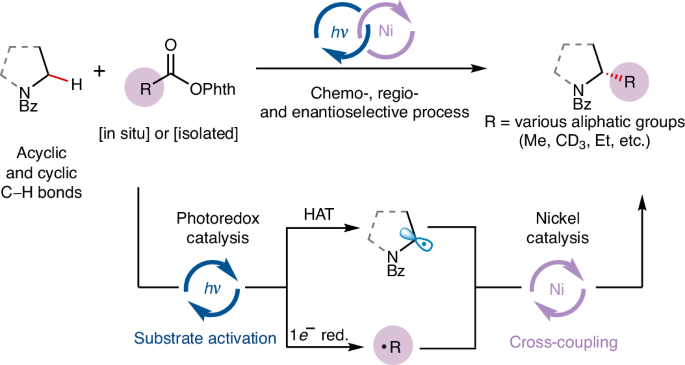
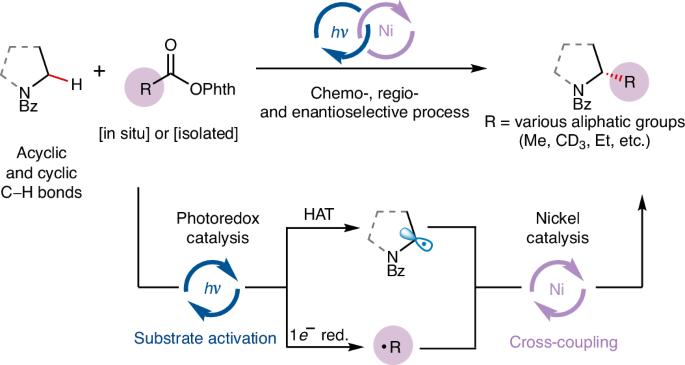
Enantioselective alkylation of α-amino C(sp3)−H bonds via photoredox and nickel catalysis
The catalytic enantioselective construction of C(sp3)−C(sp3) bonds remains a substantial challenge in organic synthesis. One particularly promising approach is the use of transition-metal-catalysed C(sp3)−H functionalization. However, a general strategy for the enantioselective alkylation of non-acidic C(sp3)−H bonds has yet to be developed. Here we present a unified platform for the enantioselective (trideutero)methylation and alkylation of α-amino C(sp3)–H bonds, using a combination of photoredox and nickel catalysis with widely available redox-active esters. This technique activates two coupling agents to form carbon-centred radicals, which are then asymmetrically coupled by a chiral nickel catalyst. This strategy is unique in its ability to separately control radical generation and cross-coupling, facilitating the use of transiently generated alkyl radicals, including highly reactive methyl radicals, in asymmetric catalysis, and thereby expediting the synthesis of enantioenriched bioactive alkaloids and offering a promising method for advancing asymmetric C(sp3)−C(sp3) bond formation. The use of a transition-metal catalyst for enantioselective alkylation of non-acidic C(sp3)–H bonds remains a challenge in organic synthesis. Now, the authors present a platform for the enantioselective (trideutero)methylation and alkylation of α-amino C(sp3)–H bonds via nickel-photoredox catalysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


