以 C-H 键为休眠体的质子传递阴离子聚合。
IF 20.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
活阴离子聚合--最常见的活聚合,也是历史最悠久的聚合--通常需要严格的无水条件和每个聚合物链一个金属引发剂。在此,我们介绍了甲基丙烯酸酯的质子传递阴离子聚合,该聚合使用酸性 C-H 键作为休眠物质,并由碱催化剂激活。聚合机理涉及增长的烯醇物种的可逆链转移或终止。弱酸性化合物(如异丁酸烷基酯)可作为引发剂或链转移剂,在笨重的钾碱催化剂存在下产生聚合物链,从而降低每条链的金属化合物比率。添加的醇类可作为可逆的终止剂来控制链的扩展。末端官能化、星形、嵌段和接枝聚合物很容易从带有 C-H 键的化合物中获得。本文章由计算机程序翻译,如有差异,请以英文原文为准。
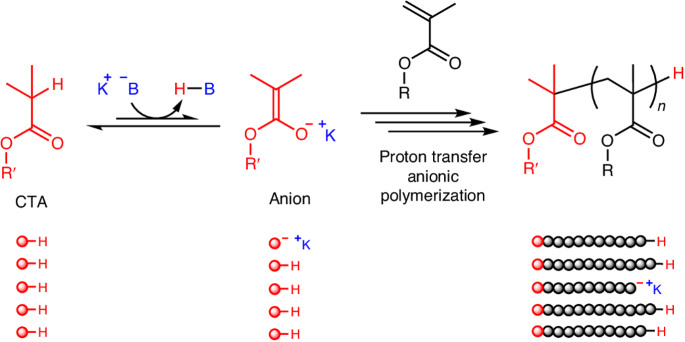
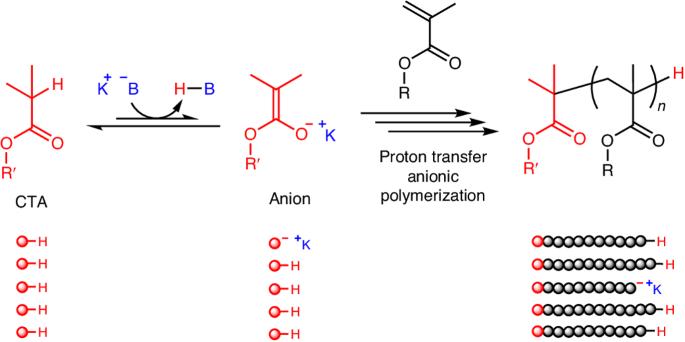
Proton transfer anionic polymerization with C–H bond as the dormant species
Living anionic polymerization—the most common living polymerization and the one with the longest history—generally requires stringent, water-free conditions and one metal initiator per polymer chain. Here we present the proton transfer anionic polymerization of methacrylates using acidic C–H bonds as the dormant species that are activated by base catalysts. The polymerization mechanism involves reversible chain transfer or termination of the growing enolate species. A weakly acidic compound, such as an alkyl isobutyrate, serves as the initiator or chain-transfer agent in the presence of a bulky potassium base catalyst to produce a polymer chain and, thereby, diminishes the metal compound per chain ratio. An added alcohol serves as a reversible terminator to tame the propagation. End-functionalized, star, block and graft polymers are easily accessible from compounds with C–H bonds. Living anionic polymerization generally requires stringent conditions and one metal initiator per polymer chain. Now it has been shown that a weakly acidic compound serves as the initiator or chain-transfer agent in the presence of a potassium base catalyst to produce a polymer chain through a proton transfer anionic polymerization mechanism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


