调谐 DNA 晶体和蛋白质-DNA 共晶体内的化学 DNA 连接
IF 4.8
Q2 NANOSCIENCE & NANOTECHNOLOGY
引用次数: 0
摘要
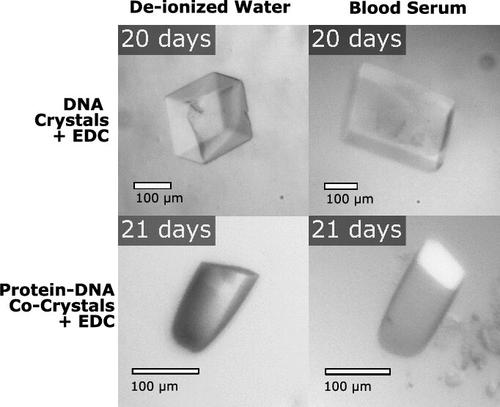
生物分子晶体可作为多种应用的材料,包括精确的客体夹持。然而,生物分子晶体在生长条件之外的溶液中很脆弱。为了充分发挥晶体作为其他分子宿主的潜力,可以通过生物共轭使晶体变得更坚固。在使用碳二亚胺 1-乙基-3-(3-(二甲基氨基)丙基)碳二亚胺(EDC)进行化学连接的前期工作基础上,我们在这里通过粘性碱基悬垂长度和支架蛋白在两类生物分子晶体(DNA 结合蛋白共晶体和纯 DNA 晶体)交联过程中的作用来研究 DNA 连接结构。这两类晶体都含有 DNA 连接点,在这些连接点上,DNA 链端对端地堆叠在一起。研究将连接产量作为粘性碱基悬垂长度和末端磷酸化状态的函数。两类晶体的最佳连接性能取决于较长的粘性悬垂长度和末端 3′磷酸化状态。值得注意的是,EDC 化学连接是在孔径太小、无法在晶体内运输连接酶的晶体中实现的。组装后的交联大大提高了 DNA 晶体和共晶体在水和血清中的稳定性。所展示的结果可能有助于含有 DNA 的晶体获得更广泛的应用,包括作为结构生物学支架。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Tuning Chemical DNA Ligation within DNA Crystals and Protein–DNA Cocrystals
Biomolecular crystals can serve as materials for a plethora of applications including precise guest entrapment. However, as grown, biomolecular crystals are fragile in solutions other than their growth conditions. For crystals to achieve their full potential as hosts for other molecules, crystals can be made stronger with bioconjugation. Building on our previous work using carbodiimide 1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide (EDC) for chemical ligation, here, we investigate DNA junction architecture through sticky base overhang lengths and the role of scaffold proteins in cross-linking within two classes of biomolecular crystals: cocrystals of DNA-binding proteins and pure DNA crystals. Both crystal classes contain DNA junctions where DNA strands stack up end-to-end. Ligation yields were studied as a function of sticky base overhang length and terminal phosphorylation status. The best ligation performance for both crystal classes was achieved with longer sticky overhangs and terminal 3′phosphates. Notably, EDC chemical ligation was achieved in crystals with pore sizes too small for intracrystal transport of ligase enzyme. Postassembly cross-linking produced dramatic stability improvements for both DNA crystals and cocrystals in water and blood serum. The results presented may help crystals containing DNA achieve broader application utility, including as structural biology scaffolds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nanoscience Au
材料科学、纳米科学-
CiteScore
4.20
自引率
0.00%
发文量
0
期刊介绍:
ACS Nanoscience Au is an open access journal that publishes original fundamental and applied research on nanoscience and nanotechnology research at the interfaces of chemistry biology medicine materials science physics and engineering.The journal publishes short letters comprehensive articles reviews and perspectives on all aspects of nanoscience and nanotechnology:synthesis assembly characterization theory modeling and simulation of nanostructures nanomaterials and nanoscale devicesdesign fabrication and applications of organic inorganic polymer hybrid and biological nanostructuresexperimental and theoretical studies of nanoscale chemical physical and biological phenomenamethods and tools for nanoscience and nanotechnologyself- and directed-assemblyzero- one- and two-dimensional materialsnanostructures and nano-engineered devices with advanced performancenanobiotechnologynanomedicine and nanotoxicologyACS Nanoscience Au also publishes original experimental and theoretical research of an applied nature that integrates knowledge in the areas of materials engineering physics bioscience and chemistry into important applications of nanomaterials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


