异型播种产生混合淀粉样蛋白多态性
IF 11.1
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
淀粉样β(Aβ)肽聚集成纤维是阿尔茨海默病(AD)发病的主要生化途径之一。为了了解纤丝种子对淀粉样蛋白聚集的整体动力学所起的作用,已经开展了大量研究。然而,在结构上或顺序上与 Aβ 不同的种子对所产生的淀粉样蛋白聚集体结构的确切影响仍有待全面了解。本文利用纳米级红外光谱探测了 Aβ42与 Aβ(16-22)和 E22Q Aβ(1-40)荷兰突变体的反平行纤丝种子聚集形成的单个聚集体的光谱面,结果表明,Aβ可形成异型或混合多态性,明显偏离其预期的平行交叉β结构。研究进一步表明,异型聚集体的形成并不局限于 Aβ 及其异构体的共聚,前者还能与α-突触核蛋白和脑蛋白裂解物形成异型纤维。这些发现凸显了AD中Aβ聚集的复杂性,并强调了探索Aβ如何与其他大脑成分相互作用的必要性,这对于开发更好的AD治疗策略至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
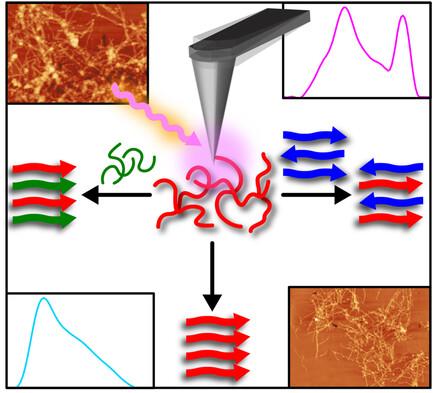
Heterotypic Seeding Generates Mixed Amyloid Polymorphs
Aggregation of the amyloid β (Aβ) peptide into fibrils represents one of the major biochemical pathways underlying the development of Alzheimer's disease (AD). Extensive studies have been carried out to understand the role of fibrillar seeds on the overall kinetics of amyloid aggregation. However, the precise effect of seeds that are structurally or sequentially different from Aβ on the structure of the resulting amyloid aggregates is yet to be fully understood. Herein, nanoscale infrared spectroscopy is used to probe the spectral facets of individual aggregates formed by aggregating Aβ42 with antiparallel fibrillar seeds of Aβ(16–22) and E22Q Aβ(1–40) Dutch mutant and it is demonstrated that Aβ can form heterotypic or mixed polymorphs that deviate significantly from its expected parallel cross β structure. It is further shown that the formation of heterotypic aggregates is not limited to the coaggregation of Aβ and its isomers, and that the former can form heterotypic fibrils with alpha-synuclein and brain protein lysates. These findings highlight the complexity of Aβ aggregation in AD and underscore the need to explore how Aβ interacts with other brain components, which is crucial for developing better therapeutic strategies for AD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
14.00
自引率
2.40%
发文量
0
期刊介绍:
Small Science is a premium multidisciplinary open access journal dedicated to publishing impactful research from all areas of nanoscience and nanotechnology. It features interdisciplinary original research and focused review articles on relevant topics. The journal covers design, characterization, mechanism, technology, and application of micro-/nanoscale structures and systems in various fields including physics, chemistry, materials science, engineering, environmental science, life science, biology, and medicine. It welcomes innovative interdisciplinary research and its readership includes professionals from academia and industry in fields such as chemistry, physics, materials science, biology, engineering, and environmental and analytical science. Small Science is indexed and abstracted in CAS, DOAJ, Clarivate Analytics, ProQuest Central, Publicly Available Content Database, Science Database, SCOPUS, and Web of Science.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


