阳离子-氧键活化和 K+/Na+ 协同促进冶炼渣中硅酸盐相解离的机理
IF 7.4
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
硅酸盐相被认为是冶炼渣的关键相,也是制约其资源利用的主要因素。因此,揭示硅酸盐相的解离机理对于提高冶炼渣的资源利用率至关重要。本研究以合成纯橄榄石的活化为研究模板,探讨硅酸盐相的活化机理。采用响应面法(RSM)验证了主要影响因素对活化效果的相关性,即矿物碱摩尔比、温度和时间。通过 DFT 计算阐明了活化过程的晶格位点置换机理和物相转化规律。结果表明硅酸盐相的活化机理包括1) OH- 吸附在阳离子位点上,导致阳氧键活化,形成羟基化合物。2) K+/Na+产生了协同效应,填补了阳离子空位,促进了阳离子的逐渐释放。本研究旨在通过深入了解硅酸盐类矿物的活化机理,为冶炼渣处理提供理论指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
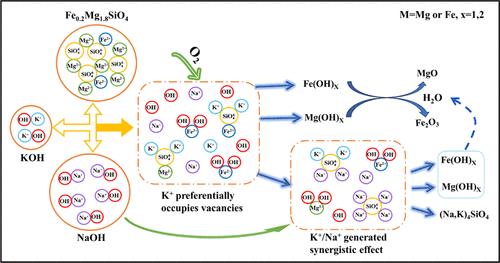
Mechanism of Cation–Oxygen Bond Activation and K+/Na+ Synergistic Promotion of Silicate Phase Dissociation in Smelting Slag
Silicate phase was identified as the key phase of smelting slag and also the main constraint for its resource utilization. Therefore, unraveling the dissociation mechanism of silicate phase was crucial for enhancing the resource utilization of smelting slag. In this study, the activation of synthesized pure olivine was utilized as a research template to investigate the silicate phase activation mechanism. The optimal conditions for olivine activation were established as olivine:NaOH:KOH = 1:5:0.5 (molar ratio), roasting temperature of 700 °C, and roasting time of 3 h. Response surface methodology (RSM) was employed to verify the correlation of the main influencing factors on the activation effect in the order of mineral alkali molar ratio > temperature > time. The lattice site substitution mechanism and the transformation law of the physical phase of the activation process were elucidated by DFT calculations. The activation mechanism of silicate phase was revealed to involve: 1) OH– adsorption on cation sites, leading to the activation of cation–oxygen bonds and the formation of the hydroxyl compounds. 2) K+/Na+ created a synergistic effect to fill the cation vacancies, facilitating the gradual release of cations. This research aims to offer theoretical guidance for smelting slag treatment by providing a deeper understanding of the activation mechanism of silicate-type minerals.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS ES&T engineering
ENGINEERING, ENVIRONMENTAL-
CiteScore
8.50
自引率
0.00%
发文量
0
期刊介绍:
ACS ES&T Engineering publishes impactful research and review articles across all realms of environmental technology and engineering, employing a rigorous peer-review process. As a specialized journal, it aims to provide an international platform for research and innovation, inviting contributions on materials technologies, processes, data analytics, and engineering systems that can effectively manage, protect, and remediate air, water, and soil quality, as well as treat wastes and recover resources.
The journal encourages research that supports informed decision-making within complex engineered systems and is grounded in mechanistic science and analytics, describing intricate environmental engineering systems. It considers papers presenting novel advancements, spanning from laboratory discovery to field-based application. However, case or demonstration studies lacking significant scientific advancements and technological innovations are not within its scope.
Contributions containing experimental and/or theoretical methods, rooted in engineering principles and integrated with knowledge from other disciplines, are welcomed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


