以过氧化二硫酸盐和对苯二酚为核心反应物的硫酸根自由基介导的化学发光:机理与环境应用
IF 7.4
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
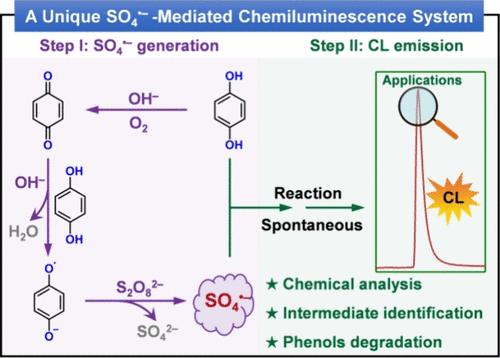
化学发光(CL)具有灵敏度高、检出限低和线性范围宽等优点,是实时定量高级氧化过程中产生的有毒污染物或中间产物的一种极具吸引力的方法。在本研究中,我们在含有对苯二酚(HQ)和过氧化二硫酸盐(PDS,S2O82-)的碱性水溶液中观察到了一种前所未有的本征 CL 现象。机理研究揭示了CL产生的两个阶段:硫酸根(SO4--)生成和CL发射。最初,高氧化性 SO4--是通过半醌自由基分解 PDS 形成的,半醌自由基来源于 HQ 与苯醌的比例反应,而苯醌是 HQ 与 OH- 在溶解氧存在下反应生成的。随后,SO4-- 会迅速将残留的 HQ 氧化为激发态发光物种,后者会返回基态,并伴随着瞬时的强光发射。值得注意的是,HQ 在发光过程中扮演着双重角色,既参与生成 SO4--,又是发光基质的前体。所提出的 CL 系统可用于痕量 HQ 的定量分析和苯酚降解动力学的实时监测。这些发现对化学分析、中间体鉴定和高级氧化过程具有重要意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Sulfate Radicals-Mediated Chemiluminescence Production with Peroxydisulfate and Hydroquinone as Coreactants: Mechanism and Environmental Applications
Chemiluminescence (CL) is an attractive method for real-time quantification of toxic contaminants or intermediates generated during advanced oxidation processes due to its high sensitivity, low detection limit, and wide linear range. In this study, we present an unprecedented intrinsic CL phenomenon observed in an alkaline aqueous solution containing hydroquinone (HQ) and peroxydisulfate (PDS, S2O82–). Mechanistic investigations unveil a two-stage process for CL production: sulfate radical (SO4•–) generation and CL emission. Initially, the highly oxidizing SO4•– are formed via the decomposition of PDS by semiquinone radicals, originating from the comproportionation reaction of HQ with benzoquinone that is generated by the reaction of HQ with OH– in the presence of dissolved oxygen. Subsequently, SO4•– promptly oxidizes the residual HQ to an excited-state light-emitting species, which returns to its ground-state, accompanied by a transient and intense light emission. Notably, HQ plays dual roles in the CL process by both participating in the generation of SO4•– and serving as the precursor of the light-emitting substrate. The proposed CL system is developed to quantify trace amounts of HQ and real-time monitor the degradation kinetics of phenols. These findings hold considerable significance in chemical analysis, intermediate identification, and advanced oxidation processes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS ES&T engineering
ENGINEERING, ENVIRONMENTAL-
CiteScore
8.50
自引率
0.00%
发文量
0
期刊介绍:
ACS ES&T Engineering publishes impactful research and review articles across all realms of environmental technology and engineering, employing a rigorous peer-review process. As a specialized journal, it aims to provide an international platform for research and innovation, inviting contributions on materials technologies, processes, data analytics, and engineering systems that can effectively manage, protect, and remediate air, water, and soil quality, as well as treat wastes and recover resources.
The journal encourages research that supports informed decision-making within complex engineered systems and is grounded in mechanistic science and analytics, describing intricate environmental engineering systems. It considers papers presenting novel advancements, spanning from laboratory discovery to field-based application. However, case or demonstration studies lacking significant scientific advancements and technological innovations are not within its scope.
Contributions containing experimental and/or theoretical methods, rooted in engineering principles and integrated with knowledge from other disciplines, are welcomed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


