开路电位法测量金属氢化物的热力学水合性
IF 2.9
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
金属氢化物普遍存在于许多催化反应中,而热力学水合度已成为了解和预测这些中间体关键氢化物转移步骤的有用参数。本报告介绍了一种开路电位法,该方法只需一个热力学参数即可确定金属氢化物的水合性。含有金属氢化物及其共轭氢化物受体以及共轭酸/碱对的溶液的开路电位 (OCP) 与已知的酸 pKa 值和 H+/H- 还原电位值相结合,可确定水合度。通过使用电位测定法获得了在乙腈溶剂中具有不同支持配体和过渡金属元素的三种氢化物络合物的水合度,从而确定了该方法的可靠性,所有结果均在之前报告值的误差范围内。讨论了该方法的优缺点,并为从业人员介绍了推荐的工作流程。本文章由计算机程序翻译,如有差异,请以英文原文为准。
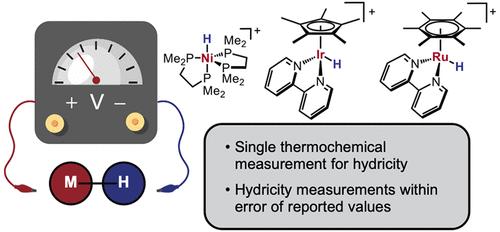
Open Circuit Potential Method for Thermodynamic Hydricity Measurements of Metal Hydrides
Metal hydrides are prevalent in many catalytic reactions, and thermodynamic hydricity has emerged as a useful parameter for understanding and predicting key hydride transfer steps with these intermediates. An open circuit potentiometry method for determining the hydricity of metal hydrides with a single thermodynamic parameter is reported. The open circuit potential (OCP) of a solution containing a metal hydride and its conjugate hydride acceptor, along with a conjugate acid/base pair, is coupled with known values for the acid pKa and H+/H– reduction potential to determine the hydricity. The reliability of the method was established by using potentiometry to obtain the hydricity of three hydride complexes with varying supporting ligands and transition metal elements in acetonitrile solvent, all within error of previously reported values. Advantages and drawbacks of this method are discussed, and a recommended workflow for practitioners is introduced.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organometallics
化学-无机化学与核化学
CiteScore
5.60
自引率
7.10%
发文量
382
审稿时长
1.7 months
期刊介绍:
Organometallics is the flagship journal of organometallic chemistry and records progress in one of the most active fields of science, bridging organic and inorganic chemistry. The journal publishes Articles, Communications, Reviews, and Tutorials (instructional overviews) that depict research on the synthesis, structure, bonding, chemical reactivity, and reaction mechanisms for a variety of applications, including catalyst design and catalytic processes; main-group, transition-metal, and lanthanide and actinide metal chemistry; synthetic aspects of polymer science and materials science; and bioorganometallic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


