面心立方碳作为第四种基本碳同素异形体,具有本征半导体和超宽带隙特性
IF 7.5
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
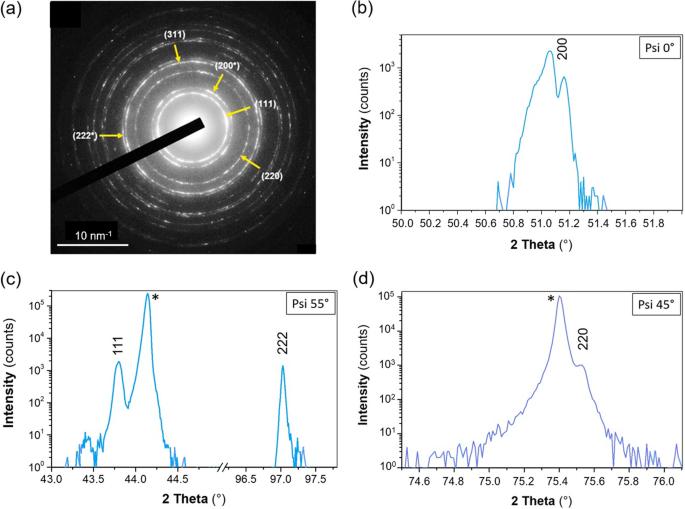
碳被认为以三种基本形式存在:金刚石、石墨/石墨烯/富勒烯和碳化烯,它们在原子轨道杂化类型上有所不同。几十年来,第四种基本碳同素异形体(面心立方(fcc)晶格)的存在一直是一个讨论的问题,尽管有明确的证据表明它可以在实验室合成并存在于自然界中。在这里,我们以金刚石外延薄膜的形式获得了这种碳同素异形体,其数量足以对其进行全面研究。这种碳材料具有 fcc 晶体结构,显示出负电子亲和性,并以价电子轨道的奇特杂化为特征。它的带隙(约 6 eV)是典型的绝缘体,而明显的电导率(约 0.1 S m-1)却随温度升高而增加,这是典型的半导体。Ab initio 计算解释了这一明显的矛盾,因为在不常见的价带结构中存在非共价共享 p 电子,从而形成带内间隙。这种碳同素异形体作为第一种具有超宽带隙的本征半导体,可以为 "碳电子学 "开辟一条新的道路。根据 s 和 p 轨道的杂化情况,已知碳有三种基本的同素异形体:金刚石、石墨/石墨烯/富勒烯和碳化烯。在这里,我们以金刚石外延薄膜的形式获得了第四种碳同素异形体,它具有面心立方晶格和奇特的原子杂化,显示出超宽带隙和半导体电子行为。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Face-centered cubic carbon as a fourth basic carbon allotrope with properties of intrinsic semiconductors and ultra-wide bandgap
Carbon is considered to exist in three basic forms: diamond, graphite/graphene/fullerenes, and carbyne, which differ in a type of atomic orbitals hybridization. Since several decades the existence of the fourth basic carbon allotropic form with the face-centered cubic (fcc) crystal lattice has been a matter of discussion despite clear evidence for its laboratory synthesis and presence in nature. Here, we obtain this carbon allotrope in form of epitaxial films on diamond in a quantity sufficient to perform their comprehensive studies. The carbon material has an fcc crystal structure, shows a negative electron affinity, and is characterized by a peculiar hybridization of the valence atomic orbitals. Its bandgap (~6 eV) is typical for insulators, whereas the noticeable electrical conductivity (~0.1 S m−1) increases with temperature, which is typical for semiconductors. Ab initio calculations explain this apparent contradiction by noncovalent sharing p-electrons present in the uncommon valence band structure comprising an intraband gap. This carbon allotrope can create a new pathway to ‘carbon electronics’ as the first intrinsic semiconductor with an ultra-wide bandgap. Carbon is known to exist in three basic allotropes depending on the hybridization of s and p orbitals: diamond, graphite/graphene/fullerenes, and carbyne. Here, a fourth carbon allotrope with a face-centered cubic crystal lattice and peculiar hybridization of atoms is obtained in the form of epitaxial films on diamond, showing an ultra-wide bandgap and semiconductor electronic behavior.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Materials
MATERIALS SCIENCE, MULTIDISCIPLINARY-
CiteScore
12.10
自引率
1.30%
发文量
85
审稿时长
17 weeks
期刊介绍:
Communications Materials, a selective open access journal within Nature Portfolio, is dedicated to publishing top-tier research, reviews, and commentary across all facets of materials science. The journal showcases significant advancements in specialized research areas, encompassing both fundamental and applied studies. Serving as an open access option for materials sciences, Communications Materials applies less stringent criteria for impact and significance compared to Nature-branded journals, including Nature Communications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


