温暖通道的凝固肖像
IF 4.3
2区 生物学
Q2 CELL BIOLOGY
引用次数: 0
摘要
为了了解蛋白质的功能,结构生物学领域广泛使用了低温电子显微镜(cryo-EM)技术,这种技术可以在玻璃体冰中嵌入蛋白质颗粒后,以原子分辨率确定结构。考虑到温度对大分子功能的深远影响,一个重要但经常被忽视的问题是冷冻颗粒与生理温度下实际蛋白质构象的关系。在最近的一项研究中,Hu 等人比较了阳离子通道 TRPM4 在 4 ℃ 和 37 ℃ 下的 "冷冻 "结构,揭示了温度如何对激活 Ca2+ 离子和其他通道调节剂的结合产生关键影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
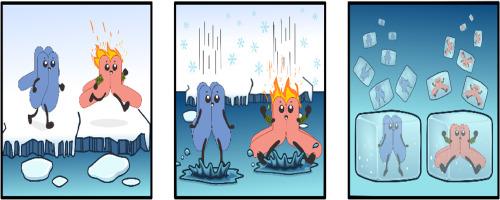
A frozen portrait of a warm channel
In order to understand protein function, the field of structural biology makes extensive use of cryogenic electron microscopy (cryo-EM), a technique that enables structure determination at atomic resolution following embedding of protein particles in vitreous ice. Considering the profound effects of temperature on macromolecule function, an important—but often neglected-question is how the frozen particles relate to the actual protein conformations at physiological temperatures. In a recent study, Hu et al. compare structures of the cation channel TRPM4 “frozen” at 4 °C versus 37 °C, revealing how temperature critically affects the binding of activating Ca2+ ions and other channel modulators.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Cell calcium
生物-细胞生物学
CiteScore
8.70
自引率
5.00%
发文量
115
审稿时长
35 days
期刊介绍:
Cell Calcium covers the field of calcium metabolism and signalling in living systems, from aspects including inorganic chemistry, physiology, molecular biology and pathology. Topic themes include:
Roles of calcium in regulating cellular events such as apoptosis, necrosis and organelle remodelling
Influence of calcium regulation in affecting health and disease outcomes
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


