B 细胞中的 PI3Kγ 促进抗体反应和抗体分泌细胞的生成
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
将幼稚和记忆 B 细胞分化为抗体分泌细胞(ASCs)是适应性免疫的一个关键特征。人们已经深入研究了支持 B 细胞生物学对磷酸肌酸 3- 激酶-δ(PI3Kδ)的要求;然而,相关的磷酸肌酸 3- 激酶-γ(PI3Kγ)复合物在 B 系细胞中的特定功能却没有得到深入研究。在本研究中,我们报告了 PI3Kγ 能促进 T 细胞依赖性抗原诱导的强大抗体反应。人类缺乏 PI3Kγ 导致的先天性免疫错误会造成广泛的体液缺陷,这促使我们研究这种激酶在抗体反应中的作用。通过使用小鼠免疫模型,我们发现 PI3Kγ 在活化的 B 细胞中以激酶活性依赖的方式发挥细胞内在功能,转导支持 ASCs 分化的转录程序所需的信号。此外,ASC命运选择与PIK3CG表达的上调相吻合,在体外CD40/细胞因子驱动活化的幼稚B细胞、toll样受体活化的记忆B细胞或人类扁桃体器官组织中,如果PI3Kγ被破坏,ASC命运选择就会受损。综上所述,我们的研究揭示了 PI3Kγ 通过整合指示 ASC 命运承诺的信号在支持体液免疫中的基本作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
PI3Kγ in B cells promotes antibody responses and generation of antibody-secreting cells
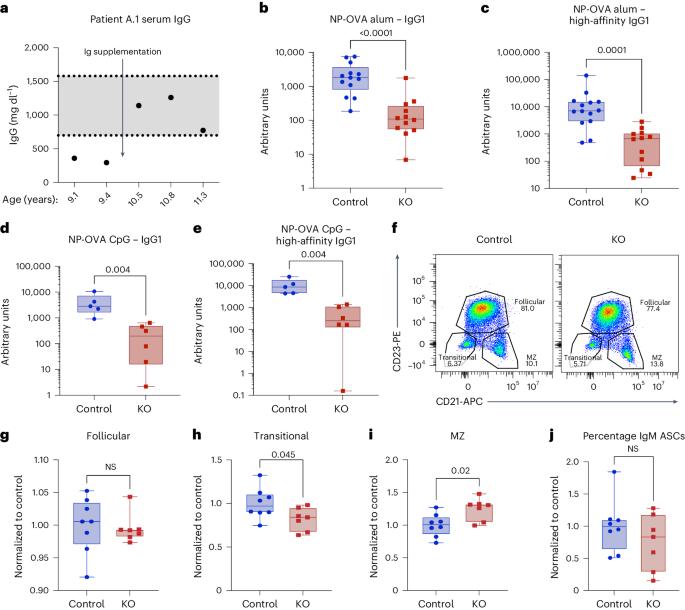
The differentiation of naive and memory B cells into antibody-secreting cells (ASCs) is a key feature of adaptive immunity. The requirement for phosphoinositide 3-kinase-delta (PI3Kδ) to support B cell biology has been investigated intensively; however, specific functions of the related phosphoinositide 3-kinase-gamma (PI3Kγ) complex in B lineage cells have not. In the present study, we report that PI3Kγ promotes robust antibody responses induced by T cell-dependent antigens. The inborn error of immunity caused by human deficiency in PI3Kγ results in broad humoral defects, prompting our investigation of roles for this kinase in antibody responses. Using mouse immunization models, we found that PI3Kγ functions cell intrinsically within activated B cells in a kinase activity-dependent manner to transduce signals required for the transcriptional program supporting differentiation of ASCs. Furthermore, ASC fate choice coincides with upregulation of PIK3CG expression and is impaired in the context of PI3Kγ disruption in naive B cells on in vitro CD40-/cytokine-driven activation, in memory B cells on toll-like receptor activation, or in human tonsillar organoids. Taken together, our study uncovers a fundamental role for PI3Kγ in supporting humoral immunity by integrating signals instructing commitment to the ASC fate. Lucas and colleagues show that differentiating B cells switch expression of PI3Kδ to PI3Kγ and that this switch is required for optimal T cell-dependent IgG antibody production in both mice and humans.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


