中枢神经系统相关巨噬细胞调节老年小鼠中风后的免疫反应
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
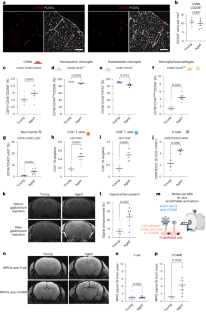
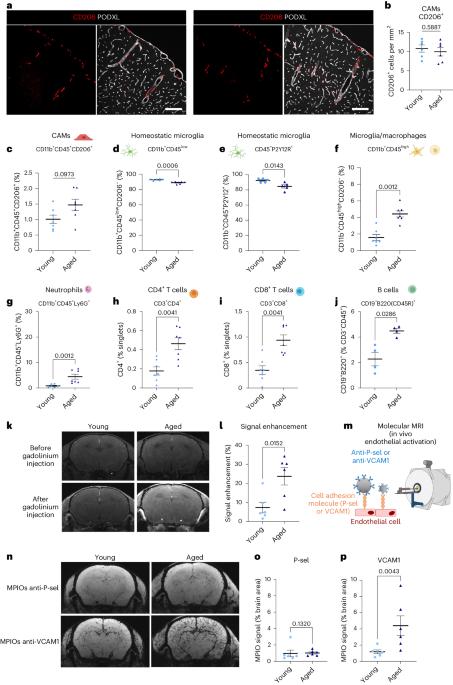
年龄是缺血性中风的一个主要不可改变的危险因素。中枢神经系统相关巨噬细胞(CAMs)是沿脑血管分布的常驻免疫细胞,位于血液循环与脑实质的交界处。我们利用临床相关的血栓栓塞性中风模型在年轻和老年雄性小鼠及相应的人类组织样本中进行研究,结果表明,在衰老过程中,CAMs 通过内皮细胞对粘附分子的特异性调节,在中风后协调免疫细胞迁移方面发挥了核心作用。CAMs 的缺失会导致中风后白细胞浸润(中性粒细胞、CD4+ 和 CD8+ T 淋巴细胞)和神经功能障碍的增加,而这些现象只发生在老年小鼠身上。主要组织相容性复合体 II 类在衰老过程中由 CAMs 过度表达,在中风的免疫反应调节中起着重要作用。我们证明,在衰老过程中,CAMs 成为神经免疫反应的核心协调者,确保中风引发的免疫反应得到长期微调。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Central nervous system-associated macrophages modulate the immune response following stroke in aged mice
Age is a major nonmodifiable risk factor for ischemic stroke. Central nervous system-associated macrophages (CAMs) are resident immune cells located along the brain vasculature at the interface between the blood circulation and the parenchyma. By using a clinically relevant thromboembolic stroke model in young and aged male mice and corresponding human tissue samples, we show that during aging, CAMs acquire a central role in orchestrating immune cell trafficking after stroke through the specific modulation of adhesion molecules by endothelial cells. The absence of CAMs provokes increased leukocyte infiltration (neutrophils and CD4+ and CD8+ T lymphocytes) and neurological dysfunction after stroke exclusively in aged mice. Major histocompatibility complex class II, overexpressed by CAMs during aging, plays a significant role in the modulation of immune responses to stroke. We demonstrate that during aging, CAMs become central coordinators of the neuroimmune response that ensure a long-term fine-tuning of the immune responses triggered by stroke. How aging influences peripheral immune cell infiltration and the role of these cells following traumatic injury of the CNS is unclear. Here, the authors show that aging transforms CNS-associated macrophages into regulators of immune cell trafficking after ischemic stroke, modulating neurological outcomes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


