表皮生长因子受体 3 驱动 Aβ 诱导的 tau 摄取。
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
淀粉样蛋白级联假说认为,淀粉样蛋白β(Aβ)有助于引发阿尔茨海默病(AD)随后的tau病理变化。然而,人们对 Aβ 促进 tau 吸收和传播的潜在机制仍然知之甚少。在这里,我们发现,先前存在的淀粉样病理学会加速细胞外 tau 被神经元吸收。通过对内含囊泡进行定量蛋白质组学分析,我们发现 Aβ 能诱导成纤维细胞生长因子受体 3(FGFR3)的内化。细胞外 tau 与 FGFR3 的细胞外结构域结合,并被 FGFR3 配体成纤维细胞生长因子 2(FGF2)内化。Aβ 可加速神经元分泌 FGF2,从而诱导 tau 连接的 FGFR3 内化。敲除海马中的 FGFR3 可减少 tau 摄取,从而减少 tau 聚集,并改善 AD 模型小鼠的记忆功能。这些数据表明,神经元中的FGFR3是一种新型tau受体,也是AD中Aβ诱导tau吸收的关键介质。本文章由计算机程序翻译,如有差异,请以英文原文为准。
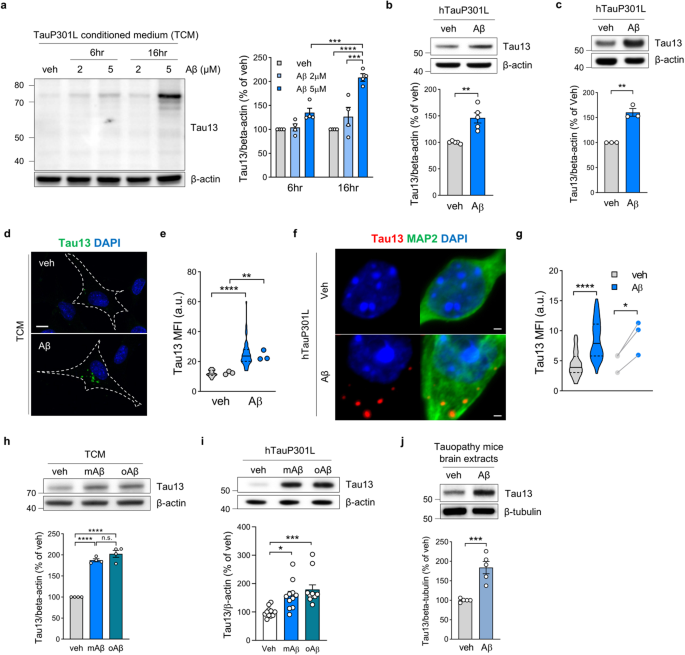
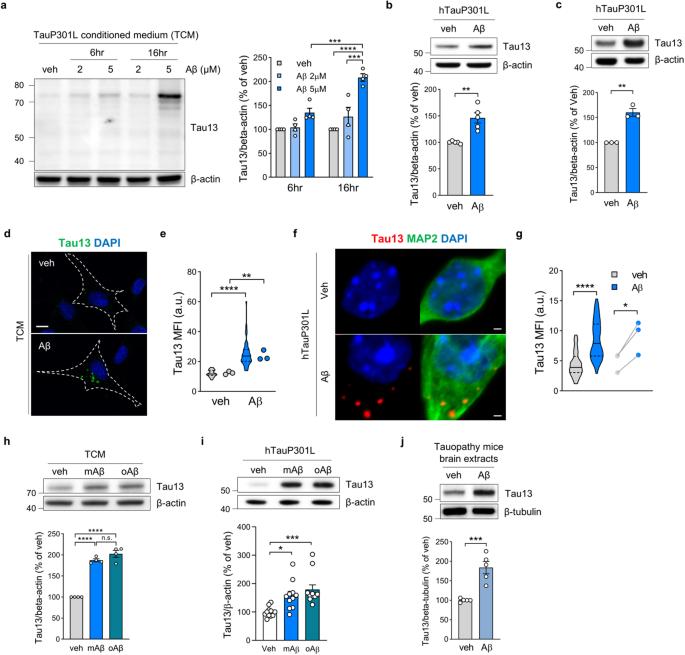
FGFR3 drives Aβ-induced tau uptake
The amyloid cascade hypothesis suggests that amyloid beta (Aβ) contributes to initiating subsequent tau pathology in Alzheimer’s disease (AD). However, the underlying mechanisms through which Aβ contributes to tau uptake and propagation remain poorly understood. Here, we show that preexisting amyloid pathology accelerates the uptake of extracellular tau into neurons. Using quantitative proteomic analysis of endocytic vesicles, we reveal that Aβ induces the internalization of fibroblast growth factor receptor 3 (FGFR3). Extracellular tau binds to the extracellular domain of FGFR3 and is internalized by the FGFR3 ligand, fibroblast growth factor 2 (FGF2). Aβ accelerates FGF2 secretion from neurons, thereby inducing the internalization of tau-attached FGFR3. Knockdown of FGFR3 in the hippocampus reduces tau aggregation by decreasing tau uptake and improving memory function in AD model mice. These data suggest FGFR3 in neurons as a novel tau receptor and a key mediator of Aβ-induced tau uptake in AD. Alzheimer’s disease (AD), the most common dementia leading to progressive memory loss and cognitive decline, is characterized by the accumulation of two pathological proteins in the brain: amyloid beta(Aβ) and tau. A recent study found that Aβ accelerates tau pathology in the brain, worsening AD. Using mice models, research showed that fibroblast growth factor receptor 3 (FGFR3) can act as tau receptor, and Aβ increases the tau uptake and aggregation by FGFR3 in brain cells. The findings suggest that decreasing FGFR3 could significantly lessen tau toxicity in brain cells. This could provide a new approach to slow down AD progression by targeting the early stages of tau accumulation. The study paves the way for potential treatments that could delay or prevent AD progression by targeting the early interaction between Aβ and tau. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


