VAMP2 在突触囊泡共缩物中合体α-突触核蛋白
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
α-突触核蛋白(α-Syn)的聚集与帕金森病的神经病理学密切相关。在生理学上,α-Syn 可促进突触小泡(SV)的聚集和可溶性 N-乙基马来酰亚胺敏感因子附着蛋白受体(SNARE)复合物的组装。然而,其潜在的结构和分子机制尚不确定,也不知道这种功能是否会影响α-Syn的病理聚集。在这里,我们发现囊泡相关膜蛋白 2(VAMP2)的并膜区--SNARE 复合物的一个驻留在 SV 上的成分--通过带电残基与 α-Syn 的羧基末端区直接相互作用,通过诱导 SV、α-Syn 和其他成分的多组分凝聚相来调节 α-Syn 在聚集 SV 和促进 SNARE 复合物组装方面的功能。此外,VAMP2 的结合还能保护 α-Syn,防止其在这些凝聚物中形成易聚集的低聚物和纤维。我们的研究结果表明,一种分子机制可以维持α-Syn的功能并防止其病理性淀粉样聚集,而淀粉样聚集的失败可能会导致帕金森病。本文章由计算机程序翻译,如有差异,请以英文原文为准。
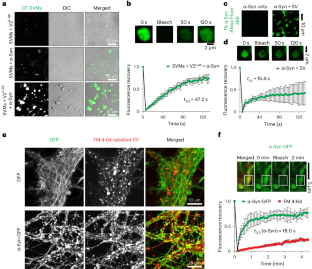
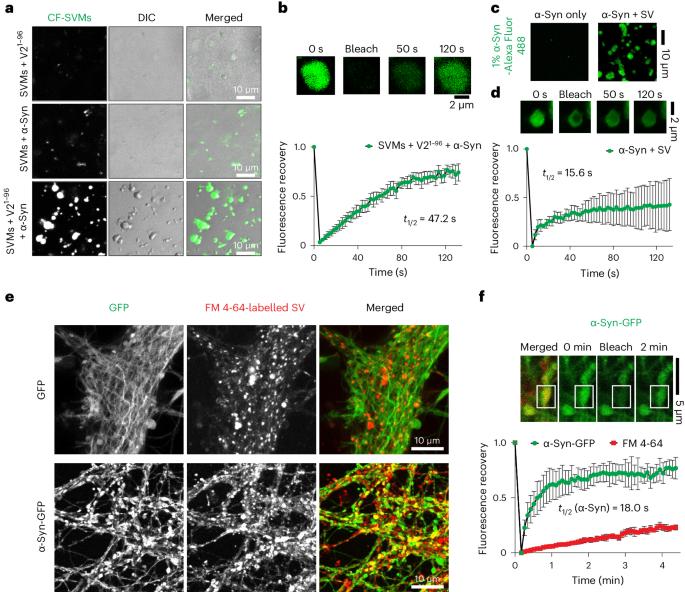
VAMP2 chaperones α-synuclein in synaptic vesicle co-condensates
α-Synuclein (α-Syn) aggregation is closely associated with Parkinson’s disease neuropathology. Physiologically, α-Syn promotes synaptic vesicle (SV) clustering and soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex assembly. However, the underlying structural and molecular mechanisms are uncertain and it is not known whether this function affects the pathological aggregation of α-Syn. Here we show that the juxtamembrane region of vesicle-associated membrane protein 2 (VAMP2)—a component of the SNARE complex that resides on SVs—directly interacts with the carboxy-terminal region of α-Syn through charged residues to regulate α-Syn’s function in clustering SVs and promoting SNARE complex assembly by inducing a multi-component condensed phase of SVs, α-Syn and other components. Moreover, VAMP2 binding protects α-Syn against forming aggregation-prone oligomers and fibrils in these condensates. Our results suggest a molecular mechanism that maintains α-Syn’s function and prevents its pathological amyloid aggregation, the failure of which may lead to Parkinson’s disease. Agarwal et al. and Wang et al. show that vesicle-associated membrane protein 2 (VAMP2) interacts with and regulates alpha-synuclein biomolecular condensation, affecting α-synuclein function, which may prevent pathological amyloid aggregation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


