抑制 Ras 可增强后部肌动蛋白收缩力驱动的细胞极化和迁移
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
Ras 作为细胞增殖的促进因子已被广泛研究,但很少有研究探讨其在迁移中的作用。为了研究 Ras 活性对细胞运动性或极性的直接和即时影响,我们重点研究了 RasGAPs(竹节虫变形虫中的 C2GAPB 和 HL-60 中性粒细胞和巨噬细胞中的 RASAL3)。在这两种细胞系统中,将各自的 RasGAP 光学招募到细胞前沿会熄灭先前存在的突起并改变迁移方向。然而,当这些各自的 RasGAP 被均匀地招募到膜上时,细胞会极化并更快地迁移,而将其定向到细胞背面则会夸大这些效应。减弱 Ras 活性的这些意想不到的结果自然会对趋化产生强烈的、依赖于环境的影响。RasGAP 介导的极化主要依赖于肌球蛋白 II 的活性,首先是细胞后部的收缩,然后是前部持续的依赖于 mTORC2 的肌动蛋白聚合。计算模拟捕捉到了这些实验结果,在模拟中,Ras 水平控制着前方和后方的促进反馈回路。抑制 Ras 活性会对细胞迁移产生反直觉效应,这一发现对未来针对致癌 Ras 的药物设计策略具有重要意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
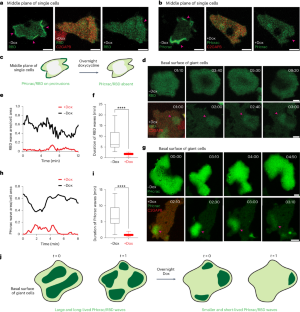
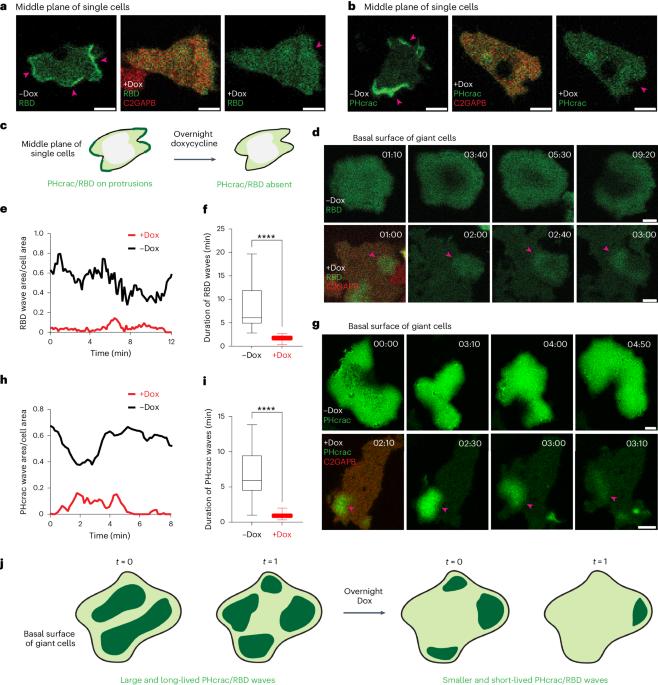
Ras suppression potentiates rear actomyosin contractility-driven cell polarization and migration
Ras has been extensively studied as a promoter of cell proliferation, whereas few studies have explored its role in migration. To investigate the direct and immediate effects of Ras activity on cell motility or polarity, we focused on RasGAPs, C2GAPB in Dictyostelium amoebae and RASAL3 in HL-60 neutrophils and macrophages. In both cellular systems, optically recruiting the respective RasGAP to the cell front extinguished pre-existing protrusions and changed migration direction. However, when these respective RasGAPs were recruited uniformly to the membrane, cells polarized and moved more rapidly, whereas targeting to the back exaggerated these effects. These unexpected outcomes of attenuating Ras activity naturally had strong, context-dependent consequences for chemotaxis. The RasGAP-mediated polarization depended critically on myosin II activity and commenced with contraction at the cell rear, followed by sustained mTORC2-dependent actin polymerization at the front. These experimental results were captured by computational simulations in which Ras levels control front- and back-promoting feedback loops. The discovery that inhibiting Ras activity can produce counterintuitive effects on cell migration has important implications for future drug-design strategies targeting oncogenic Ras. Lin, Pal, et al. report a role for localized activation of Ras activity in promoting cell polarity and motility.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


