通过单细胞多组学透视线粒体遗传学
IF 29
1区 生物学
Q1 GENETICS & HEREDITY
引用次数: 0
摘要
线粒体携带自身的遗传信息,编码对细胞呼吸和新陈代谢至关重要的蛋白质编码基因和翻译机制。尽管线粒体体积小,但由于其细胞拷贝数的变化、非孟德尔遗传模式和突变倾向,使用批量测序方法研究线粒体基因组、其自然遗传变异和分子表型一直是一项挑战。在此,我们将重点介绍旨在捕捉单个细胞线粒体遗传变异的新兴策略,以便对原代人类细胞和临床标本进行系谱追踪和线粒体遗传学研究。我们回顾了围绕单细胞线粒体基因组测序及其与功能基因组读数整合的最新进展,包括利用体细胞线粒体 DNA 变异作为克隆标记,从而解析复杂人体组织中的细胞群体动态。最后,我们将讨论如何利用单细胞全线粒体基因组测序方法来研究线粒体遗传学及其对细胞异质性和疾病的贡献。本文章由计算机程序翻译,如有差异,请以英文原文为准。
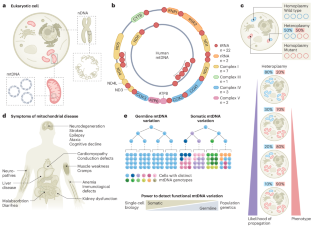
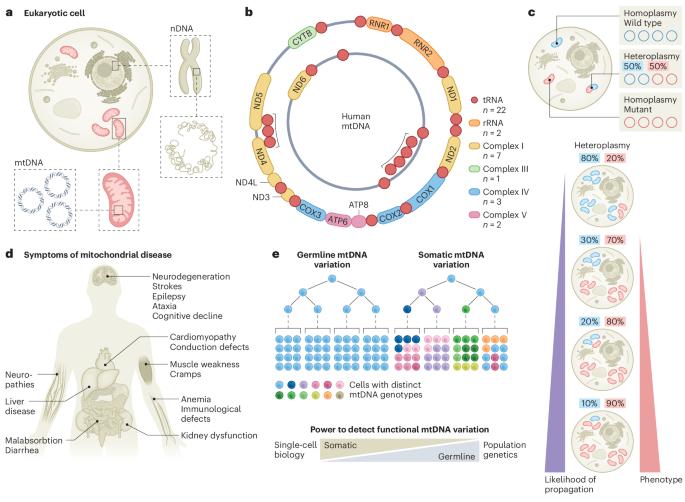
Mitochondrial genetics through the lens of single-cell multi-omics
Mitochondria carry their own genetic information encoding for a subset of protein-coding genes and translational machinery essential for cellular respiration and metabolism. Despite its small size, the mitochondrial genome, its natural genetic variation and molecular phenotypes have been challenging to study using bulk sequencing approaches, due to its variation in cellular copy number, non-Mendelian modes of inheritance and propensity for mutations. Here we highlight emerging strategies designed to capture mitochondrial genetic variation across individual cells for lineage tracing and studying mitochondrial genetics in primary human cells and clinical specimens. We review recent advances surrounding single-cell mitochondrial genome sequencing and its integration with functional genomic readouts, including leveraging somatic mitochondrial DNA mutations as clonal markers that can resolve cellular population dynamics in complex human tissues. Finally, we discuss how single-cell whole mitochondrial genome sequencing approaches can be utilized to investigate mitochondrial genetics and its contribution to cellular heterogeneity and disease. This Review discusses emerging technologies and insights from mitochondrial DNA variant profiling obtained by single-cell multi-omics, demonstrating how the powerhouse of the cell may record clonal lineages and contribute to disease heterogeneity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature genetics
生物-遗传学
CiteScore
43.00
自引率
2.60%
发文量
241
审稿时长
3 months
期刊介绍:
Nature Genetics publishes the very highest quality research in genetics. It encompasses genetic and functional genomic studies on human and plant traits and on other model organisms. Current emphasis is on the genetic basis for common and complex diseases and on the functional mechanism, architecture and evolution of gene networks, studied by experimental perturbation.
Integrative genetic topics comprise, but are not limited to:
-Genes in the pathology of human disease
-Molecular analysis of simple and complex genetic traits
-Cancer genetics
-Agricultural genomics
-Developmental genetics
-Regulatory variation in gene expression
-Strategies and technologies for extracting function from genomic data
-Pharmacological genomics
-Genome evolution
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


