非对映发散亲核-亲核烯烃氯氟化反应
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
烯烃的选择性异卤化反应为广泛的化学应用提供了有用的基础材料。与同族二卤化反应不同,选择性异族二卤化反应,尤其是氟卤化反应,还没有得到充分发展。目前的方法是将亲电卤素源与亲核卤素源结合起来,这必然会导致反加成,而且只有使用高度活化的烯类才能实现区域选择性。在这里,我们介绍了另一种亲核-亲核方法,它能以高度区域、化学和非对映选择性的方式在未活化的烯烃上添加氯离子和氟离子。在反应机理中发现了一个奇特的开关,它能引发非对映选择性的完全逆转,从而促进反加成或合加成。详细的机理研究表明,选择性以及合成和反非对映异构体之间的转换是基于不同的活性碘烷以及两种卤化物中哪一种先加成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
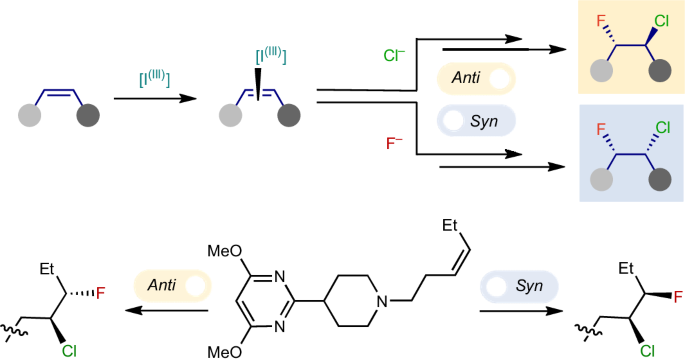
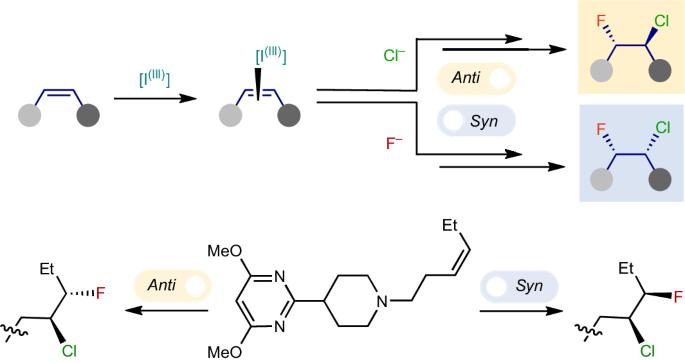
Diastereodivergent nucleophile–nucleophile alkene chlorofluorination
The selective hetero-dihalogenation of alkenes provides useful building blocks for a broad range of chemical applications. Unlike homo-dihalogenation, selective hetero-dihalogenation reactions, especially fluorohalogenation, are underdeveloped. Current approaches combine an electrophilic halogen source with a nucleophilic halogen source, which necessarily leads to anti-addition, and regioselectivity has only been achieved using highly activated alkenes. Here we describe an alternative, nucleophile–nucleophile approach that adds chloride and fluoride ions over unactivated alkenes in a highly regio-, chemo- and diastereoselective manner. A curious switch in the reaction mechanism was discovered, which triggers a complete reversal of the diastereoselectivity to promote either anti- or syn-addition. The conditions are demonstrated on an array of pharmaceutically relevant compounds, and detailed mechanistic studies reveal the selectivity and the switch between the syn- and anti-diastereomers are based on different active iodanes and which of the two halides adds first. Unlike homo-dihalogenation, selective hetero-dihalogenation reactions are underdeveloped. Now an oxidative alkene hetero-dihalogenation reaction adds chloride and fluoride ions over unactivated alkenes with high regio-, chemo- and diastereoselectivity. A switch in the mechanism triggers a reversal of the diastereoselectivity to promote either anti- or syn-addition.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


