非编码 RNA 可调控 Krm1 和 Dkk2 的表达,从而协同影响主动脉瓣病变。
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
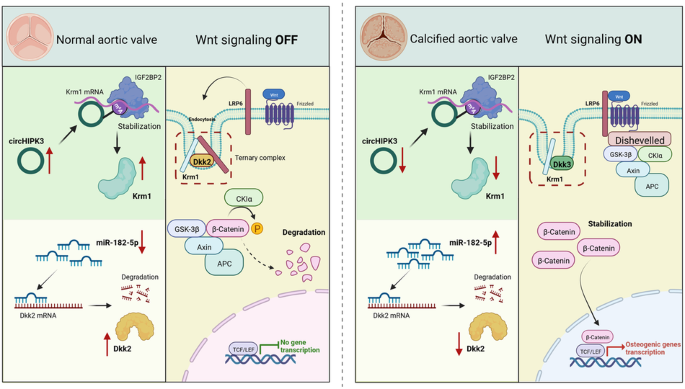
钙化性主动脉瓣病(CAVD)正成为一个日益重要的全球性医疗问题,但却缺乏有效的药物治疗方法。非编码 RNA 在心血管疾病的进展中起着关键作用,但它们与 CAVD 的关系仍不清楚。测序数据显示,许多非编码 RNA 在正常主动脉瓣和钙化主动脉瓣中的表达存在差异,其中 circHIPK3 和 miR-182-5p 的表达差异显著。过表达 circHIPK3 能改善 CAVD 小鼠模型中主动脉瓣的病变。体外实验证明,circHIPK3能抑制主动脉瓣间质细胞的成骨反应。从机理上讲,DEAD-box螺旋酶5(DDX5)招募甲基转移酶3(METTL3)来促进circHIPK3的N6-甲基腺苷(m6A)修饰。此外,m6A修饰的circHIPK3会增加Kremen1(Krm1)mRNA的稳定性,而Krm1是Wnt/β-catenin通路的负调控因子。此外,miR-182-5p 还能抑制 Krm1 的配体 Dickkopf2(Dkk2)的表达,减弱 Krm1 介导的 Wnt 信号转导抑制作用。Wnt信号通路的激活在很大程度上促进了主动脉瓣的钙化。我们的研究描述了 Krm1-Dkk2 轴在抑制主动脉瓣 Wnt 信号传导中的作用,并表明非编码 RNA 是这一过程的上游调节因子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Noncoding RNA regulates the expression of Krm1 and Dkk2 to synergistically affect aortic valve lesions
Calcific aortic valve disease (CAVD) is becoming an increasingly important global medical problem, but effective pharmacological treatments are lacking. Noncoding RNAs play a pivotal role in the progression of cardiovascular diseases, but their relationship with CAVD remains unclear. Sequencing data revealed differential expression of many noncoding RNAs in normal and calcified aortic valves, with significant differences in circHIPK3 and miR-182-5p expression. Overexpression of circHIPK3 ameliorated aortic valve lesions in a CAVD mouse model. In vitro experiments demonstrated that circHIPK3 inhibits the osteogenic response of aortic valve interstitial cells. Mechanistically, DEAD-box helicase 5 (DDX5) recruits methyltransferase 3 (METTL3) to promote the N6-methyladenosine (m6A) modification of circHIPK3. Furthermore, m6A-modified circHIPK3 increases the stability of Kremen1 (Krm1) mRNA, and Krm1 is a negative regulator of the Wnt/β-catenin pathway. Additionally, miR-182-5p suppresses the expression of Dickkopf2 (Dkk2), the ligand of Krm1, and attenuates the Krm1-mediated inhibition of Wnt signaling. Activation of the Wnt signaling pathway significantly contributes to the promotion of aortic valve calcification. Our study describes the role of the Krm1-Dkk2 axis in inhibiting Wnt signaling in aortic valves and suggests that noncoding RNAs are upstream regulators of this process. Calcific aortic valve disease (CAVD, a common heart condition) currently has no effective treatments. This research aimed to examine the role of non-coding RNAs (molecules that control gene activity) in CAVD, particularly circHIPK3 and miR-182-5p. Experiments were conducted on human heart valve cells and mice, showing that circHIPK3 can prevent heart valve hardening, while miR-182-5p can trigger a process that encourages hardening. The research also discovered a protein, Krm1, that can stop this hardening process. These results suggest that focusing on these non-coding RNAs and proteins could offer a new way to treat CAVD. However, more research is needed to fully comprehend these processes and their potential treatment implications. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


