多发性骨髓瘤的蛋白质基因组图谱揭示了疾病生物学和治疗机会。
IF 23.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
多发性骨髓瘤(MM)是一种骨髓浆细胞恶性肿瘤。尽管治疗手段不断进步,但多发性骨髓瘤仍无法治愈,因此亟需更好的风险分层和新疗法。MM 的蛋白质组以前从未被系统评估过,除了基因和转录组研究外,蛋白质组还有可能揭示疾病的生物学特性并改善预后。在这里,我们提供了一项全面的多组学分析,包括基于深度串联质量标签的全局(磷)定量蛋白质组学、RNA测序和纳米孔DNA测序,分析对象是138例原发性浆细胞恶性肿瘤患者,包括未经治疗的MM、浆细胞白血病和恶性肿瘤前意义未定的单克隆性腺病以及健康对照组。我们发现,与健康浆细胞相比,恶性浆细胞的(磷酸化)蛋白质组高度失调,并由染色体改变和转录后调控共同决定。研究发现了一种预后蛋白特征,它与MM的侵袭性疾病相关,与已确定的风险因素无关。与功能遗传学和单细胞 RNA 测序相结合,发现了浆细胞恶性肿瘤中一般的和基因亚型特异性的失调蛋白和通路,其中包括潜在的(免疫)疗法靶点。我们的研究证明了蛋白质基因组学在癌症中的潜力,并为研究 MM 的蛋白质调控和新的治疗方法提供了易于获取的资源。本文章由计算机程序翻译,如有差异,请以英文原文为准。
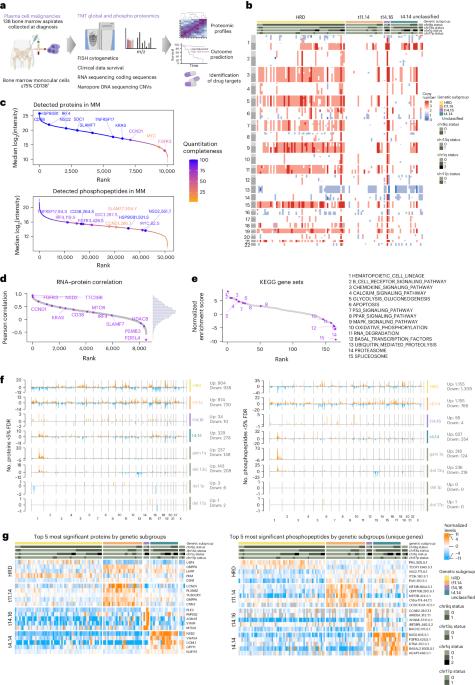
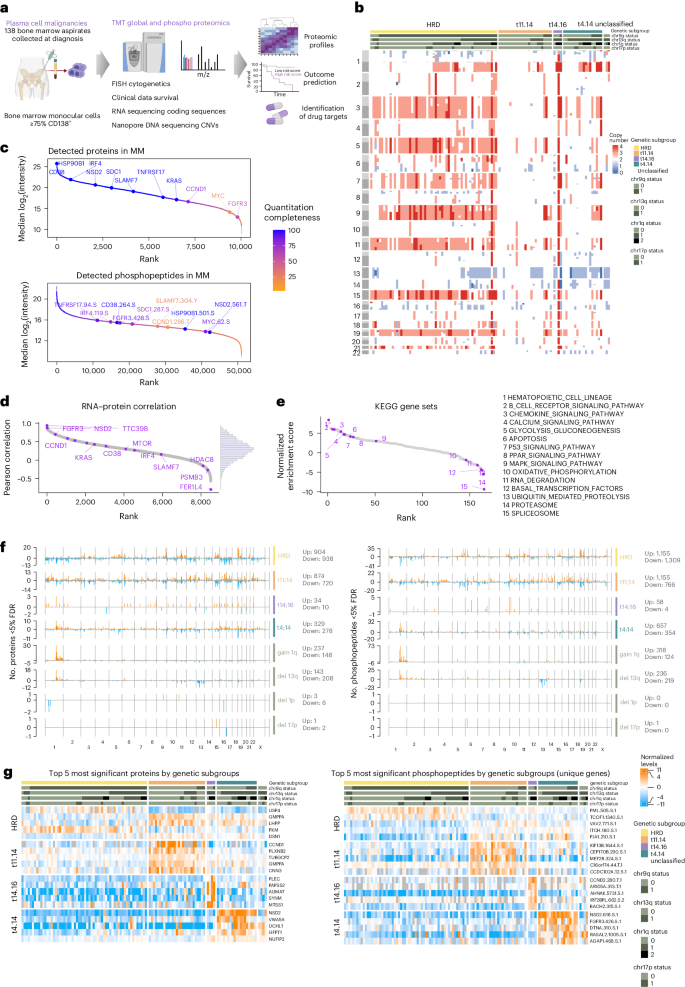
The proteogenomic landscape of multiple myeloma reveals insights into disease biology and therapeutic opportunities
Multiple myeloma (MM) is a plasma cell malignancy of the bone marrow. Despite therapeutic advances, MM remains incurable, and better risk stratification as well as new therapies are therefore highly needed. The proteome of MM has not been systematically assessed before and holds the potential to uncover insight into disease biology and improved prognostication in addition to genetic and transcriptomic studies. Here we provide a comprehensive multiomics analysis including deep tandem mass tag-based quantitative global (phospho)proteomics, RNA sequencing, and nanopore DNA sequencing of 138 primary patient-derived plasma cell malignancies encompassing treatment-naive MM, plasma cell leukemia and the premalignancy monoclonal gammopathy of undetermined significance, as well as healthy controls. We found that the (phospho)proteome of malignant plasma cells are highly deregulated as compared with healthy plasma cells and is both defined by chromosomal alterations as well as posttranscriptional regulation. A prognostic protein signature was identified that is associated with aggressive disease independent of established risk factors in MM. Integration with functional genetics and single-cell RNA sequencing revealed general and genetic subtype-specific deregulated proteins and pathways in plasma cell malignancies that include potential targets for (immuno)therapies. Our study demonstrates the potential of proteogenomics in cancer and provides an easily accessible resource for investigating protein regulation and new therapeutic approaches in MM. Krönke and colleagues present a multiomic resource of plasma cell malignancies, including multiple myeloma, that comprises phosphoproteomics, RNA and DNA sequencing and provides insights into cancer type biology and candidate therapeutic targets.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature cancer
Medicine-Oncology
CiteScore
31.10
自引率
1.80%
发文量
129
期刊介绍:
Cancer is a devastating disease responsible for millions of deaths worldwide. However, many of these deaths could be prevented with improved prevention and treatment strategies. To achieve this, it is crucial to focus on accurate diagnosis, effective treatment methods, and understanding the socioeconomic factors that influence cancer rates.
Nature Cancer aims to serve as a unique platform for sharing the latest advancements in cancer research across various scientific fields, encompassing life sciences, physical sciences, applied sciences, and social sciences. The journal is particularly interested in fundamental research that enhances our understanding of tumor development and progression, as well as research that translates this knowledge into clinical applications through innovative diagnostic and therapeutic approaches. Additionally, Nature Cancer welcomes clinical studies that inform cancer diagnosis, treatment, and prevention, along with contributions exploring the societal impact of cancer on a global scale.
In addition to publishing original research, Nature Cancer will feature Comments, Reviews, News & Views, Features, and Correspondence that hold significant value for the diverse field of cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


