人工智能辅助检测前哨淋巴结乳腺癌转移的临床实施:CONFIDENT-B 单中心非随机临床试验。
IF 23.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
病理学家对乳腺癌(BC)转移的前哨淋巴结(SN)进行评估是一项指导治疗的工作,但由于免疫组化(IHC)在形态学阴性病例中的表现,这项工作耗费大量人力和财力。这项非随机、单中心临床试验(国际标准随机对照试验编号:14323711)评估了人工智能(AI)辅助工作流程在保持诊断安全标准的前提下检测SN中乳腺癌转移灶的疗效。从2022年9月到2023年5月,190份SN标本连续入组,每两周分配到干预组(n = 100)或对照组(n = 90)。在干预组和对照组中,SN标本苏木精-伊红切片的数字全切片图像均由病理专家进行评估,病理专家在 "转移检测 "应用程序(Visiopharm)的协助下进行评估。我们的主要终点显示,在人工智能辅助下,病理学家使用 IHC 的调整后相对风险明显降低(0.680,95% 置信区间:0.347-0.878),从而节省了约 3000 欧元的成本。次要终点显示,人工智能辅助病理学家显著缩短了时间,提高了多达 30% 的灵敏度。这项试验证明了人工智能辅助的安全性以及节约成本和时间的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
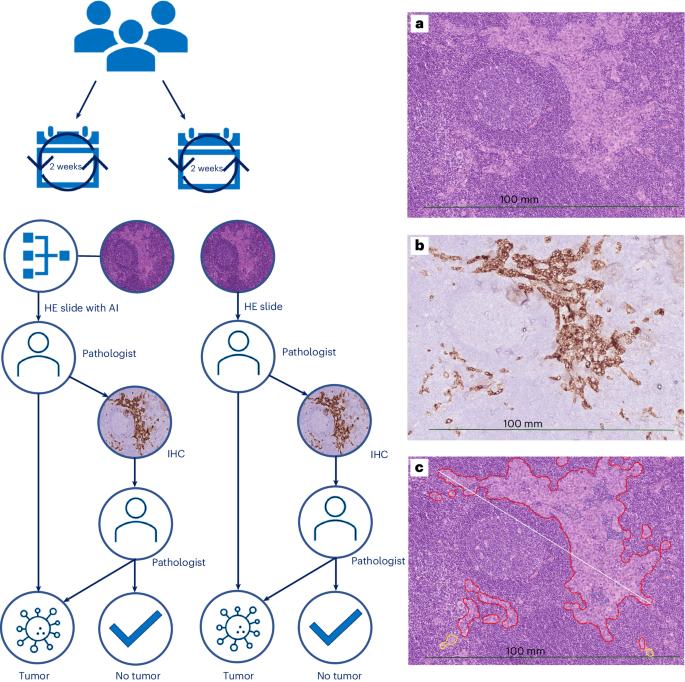
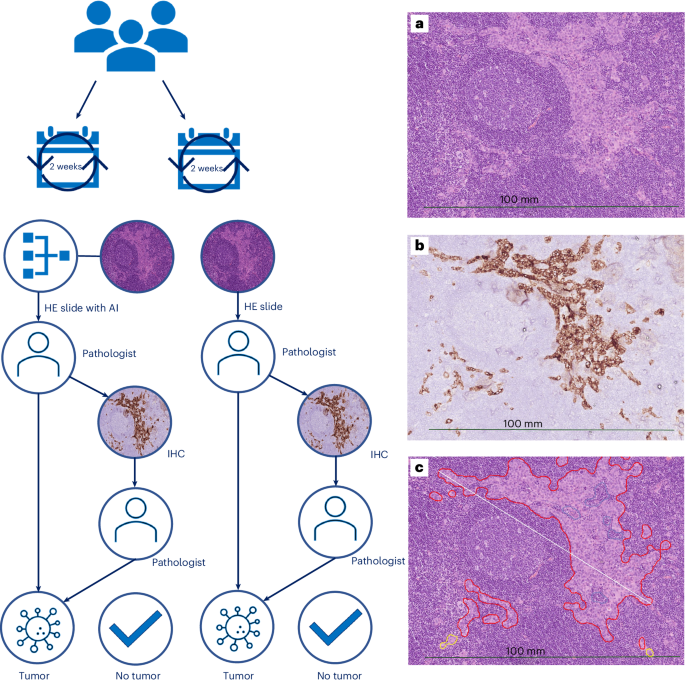
Clinical implementation of artificial-intelligence-assisted detection of breast cancer metastases in sentinel lymph nodes: the CONFIDENT-B single-center, non-randomized clinical trial
Pathologists’ assessment of sentinel lymph nodes (SNs) for breast cancer (BC) metastases is a treatment-guiding yet labor-intensive and costly task because of the performance of immunohistochemistry (IHC) in morphologically negative cases. This non-randomized, single-center clinical trial (International Standard Randomized Controlled Trial Number:14323711) assessed the efficacy of an artificial intelligence (AI)-assisted workflow for detecting BC metastases in SNs while maintaining diagnostic safety standards. From September 2022 to May 2023, 190 SN specimens were consecutively enrolled and allocated biweekly to the intervention arm (n = 100) or control arm (n = 90). In both arms, digital whole-slide images of hematoxylin–eosin sections of SN specimens were assessed by an expert pathologist, who was assisted by the ‘Metastasis Detection’ app (Visiopharm) in the intervention arm. Our primary endpoint showed a significantly reduced adjusted relative risk of IHC use (0.680, 95% confidence interval: 0.347–0.878) for AI-assisted pathologists, with subsequent cost savings of ~3,000 €. Secondary endpoints showed significant time reductions and up to 30% improved sensitivity for AI-assisted pathologists. This trial demonstrates the safety and potential for cost and time savings of AI assistance. Van Dooijeweert et al. conducted a prospective study on the clinical implementation of artificial-intelligence-assisted detection of sentinel lymph node metastasis in persons with breast cancer and report on its effects, including on time and cost.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature cancer
Medicine-Oncology
CiteScore
31.10
自引率
1.80%
发文量
129
期刊介绍:
Cancer is a devastating disease responsible for millions of deaths worldwide. However, many of these deaths could be prevented with improved prevention and treatment strategies. To achieve this, it is crucial to focus on accurate diagnosis, effective treatment methods, and understanding the socioeconomic factors that influence cancer rates.
Nature Cancer aims to serve as a unique platform for sharing the latest advancements in cancer research across various scientific fields, encompassing life sciences, physical sciences, applied sciences, and social sciences. The journal is particularly interested in fundamental research that enhances our understanding of tumor development and progression, as well as research that translates this knowledge into clinical applications through innovative diagnostic and therapeutic approaches. Additionally, Nature Cancer welcomes clinical studies that inform cancer diagnosis, treatment, and prevention, along with contributions exploring the societal impact of cancer on a global scale.
In addition to publishing original research, Nature Cancer will feature Comments, Reviews, News & Views, Features, and Correspondence that hold significant value for the diverse field of cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


