在设计光电化学材料时过分强调绝对带边能量
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
半导体的绝对带边电位及其相对于溶液氧化还原电位的位置经常被用作光电化学装置和微粒光催化剂的设计原则。在这里,我们要说明的是,这些标准并非必要,它们限制了对可能推动光电化学、光化学和光催化领域发展的材料的探索。我们讨论了带边能量并非单一参数,而是会随着 pH 值、电解质类型和表面化学而变化。电子和空穴的自由能,而不是溶液氧化还原偶的自由能,决定了整个反应的自发性,从而决定了反应活性。即使相关的电解质氧化还原电位出现在带隙之外,通过半导体表面电子电荷的反转或积累,也会产生有利的电荷转移动力学。这一讨论为单电子和多电子氧化还原反应(如 H2 演化、H2O 氧化和 CO2 还原)的光催化系统工程设计原则提供了参考。本文章由计算机程序翻译,如有差异,请以英文原文为准。
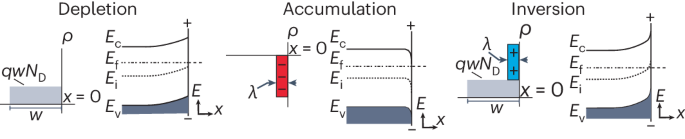
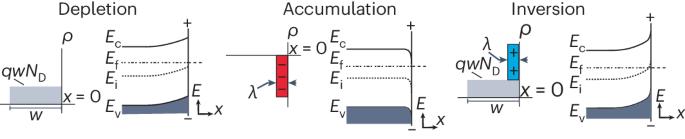
Absolute band-edge energies are over-emphasized in the design of photoelectrochemical materials
The absolute band-edge potentials of semiconductors and their positions relative to solution redox potentials are often invoked as design principles for photoelectrochemical devices and particulate photocatalysts. Here we show that these criteria are not necessary and limit the exploration of materials that may advance the fields of photoelectrochemistry, photochemistry and photocatalysis. We discuss how band-edge energies are not singular parameters and instead shift with pH, electrolyte type and surface chemistry. The free energies of electrons and holes, rather than those of solution redox couples, dictate overall reaction spontaneity and thus reactivity. Favourable charge-transfer kinetics can occur even when the relevant electrolyte redox potential(s) appear outside the bandgap, enabled by the inversion or accumulation of electronic charge at the semiconductor surface. This discussion informs design principles for photocatalytic systems engineering for both one-electron and multi-electron redox reactions (for example, H2 evolution, H2O oxidation and CO2 reduction). The absolute position of band edges is widely considered an indispensable design principle for selection of appropriate semiconductors for a given photo(electro)catalytic reaction. In this Perspective, the authors re-examine this idea from a viewpoint of semiconductor physics and make the case that alignment of band edges with chemical redox potentials is of limited importance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


