泌乳期奶牛胃肠道转录组特征分析揭示了牛奶合成的分子适应性。
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
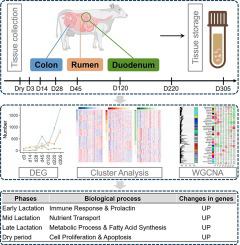
在泌乳期,奶牛的消化道需要进行重大调整,以满足产奶所需的更多营养。当我们试图通过选择压力来改善牛奶相关性状时,了解瘤胃上皮、小肠上皮和结肠组织的生物功能对牛奶合成营养需求变化所驱动的生理状态变化的响应至关重要。在这项研究中,我们从五头荷斯坦奶牛的三种组织(结肠上皮、十二指肠上皮和瘤胃上皮)中获得了 108 个转录组图谱,时间跨度从泌乳初期、中期、后期到干乳期的八个时间点。平均有 97.06% 的读数成功映射到参考基因组序列 ARS-UCD1.2。我们分析了多个时期的 27,607 个基因表达模式,从而可以直接比较不同泌乳阶段(包括泌乳早期和泌乳高峰期)组织内和组织间的基因表达。我们在结肠、十二指肠和瘤胃中分别发现了 1645、813 和 2187 个特定阶段的基因,这些基因在不同组织中富集了共同或特定的生物功能。时间序列分析将每个组织内的表达基因分为四个群组。此外,在对这三种组织进行综合分析时,还发现了 36 个表达相似的基因簇。通过整合基因共表达分析、功能富集和细胞类型解卷积等其他综合方法,我们对牛的泌乳期有了深刻的认识,揭示了胃肠道的组织特异性特征,并阐明了泌乳期营养吸收、免疫调节和乳汁合成的细胞过程所涉及的错综复杂的分子适应性。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Transcriptomic profiling of gastrointestinal tracts in dairy cattle during lactation reveals molecular adaptations for milk synthesis
During lactation, dairy cattle’s digestive tract requires significant adaptations to meet the increased nutrient demands for milk production. As we attempt to improve milk-related traits through selective pressure, it is crucial to understand the biological functions of the epithelia of the rumen, small intestine, and colonic tissues in response to changes in physiological state driven by changes in nutrient demands for milk synthesis. In this study, we obtained a total of 108 transcriptome profiles from three tissues (epithelia of the colon, duodenum, and rumen) of five Holstein cows, spanning eight time points from the early, mid, late lactation periods to the dry period. On average 97.06% of reads were successfully mapped to the reference genome assembly ARS-UCD1.2. We analyzed 27,607 gene expression patterns at multiple periods, enabling direct comparisons within and among tissues during different lactation stages, including early and peak lactation. We identified 1645, 813, and 2187 stage-specific genes in the colon, duodenum, and rumen, respectively, which were enriched for common or specific biological functions among different tissues. Time series analysis categorized the expressed genes within each tissue into four clusters. Furthermore, when the three tissues were analyzed collectively, 36 clusters of similarly expressed genes were identified. By integrating other comprehensive approaches such as gene co-expression analyses, functional enrichment, and cell type deconvolution, we gained profound insights into cattle lactation, revealing tissue-specific characteristics of the gastrointestinal tract and shedding light on the intricate molecular adaptations involved in nutrient absorption, immune regulation, and cellular processes for milk synthesis during lactation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


