ERGIC定位的Rabs在TMED10介导的非常规蛋白质分泌中发挥双重作用
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
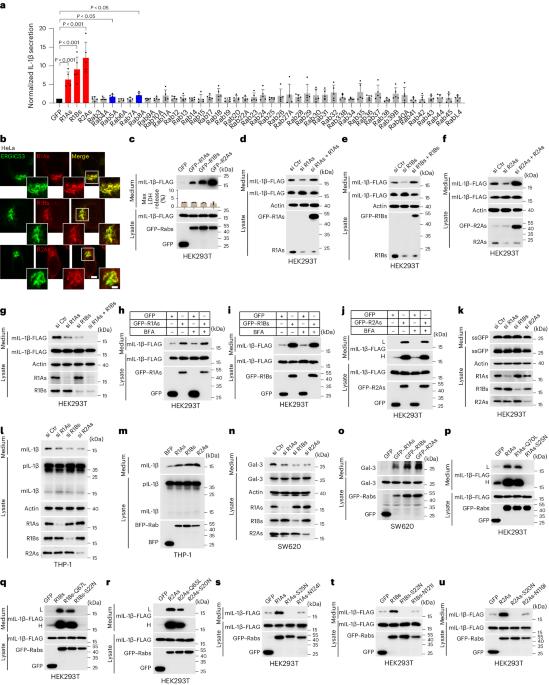
货物跨膜转运是分泌的一个重要方面。在常规分泌过程中,装有信号肽的蛋白质进入内质网(ER),而缺乏信号肽的货物则转运到ER-高尔基体中间区室(ERGIC),这一过程被称为非常规蛋白质分泌(UcPS)。UcPS中ERGIC的调控事件尚不清楚。在这里,我们揭示了ERGIC定位的小GTP酶Rab1(Rab1A和Rab1B)和Rab2A参与了通过ERGIC上的TMED10调控UcPS货物运输的过程。Rab1增强了TMED10转运体的活性,促进货物转运到ERGIC,而Rab2A则与KIF5B合作,调节ERGIC的区隔,建立UcPS特异性区隔。这项研究强调了ERGIC定位的Rabs在管理货物转运和明确ERGIC在UcPS中的功能方面的关键作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A dual role of ERGIC-localized Rabs in TMED10-mediated unconventional protein secretion
Cargo translocation across membranes is a crucial aspect of secretion. In conventional secretion signal peptide-equipped proteins enter the endoplasmic reticulum (ER), whereas a subset of cargo lacking signal peptides translocate into the ER–Golgi intermediate compartment (ERGIC) in a process called unconventional protein secretion (UcPS). The regulatory events at the ERGIC in UcPS are unclear. Here we reveal the involvement of ERGIC-localized small GTPases, Rab1 (Rab1A and Rab1B) and Rab2A, in regulating UcPS cargo transport via TMED10 on the ERGIC. Rab1 enhances TMED10 translocator activity, promoting cargo translocation into the ERGIC, whereas Rab2A, in collaboration with KIF5B, regulates ERGIC compartmentalization, establishing a UcPS-specific compartment. This study highlights the pivotal role of ERGIC-localized Rabs in governing cargo translocation and specifying the ERGIC’s function in UcPS. Sun, Tao, Han et al. identify the Rabs involved in unconventional protein secretion mediated by TMED10 and in ER–Golgi intermediate compartment organization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


