SnCl2 催化的多组分偶联:使用多聚甲醛作为 C1 原料合成 1,3-恶唑烷衍生物。
IF 2.9
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
SnCl2 催化了苯胺、环氧化物和多聚甲醛的三组分偶联反应,从而合成了 1,3-恶唑烷衍生物。该反应简单,不需要任何添加剂、碱或氧化剂,在中等温度下进行,具有良好的官能团耐受性。利用多聚甲醛作为亚甲基源的范围进一步扩大到使用相同催化剂合成苯并噻唑和 4,4'-亚甲基双(N,N-二甲基苯胺)。根据对照实验提出了一种催化途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
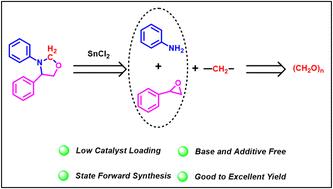
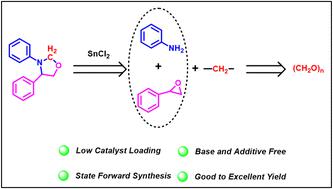
SnCl2-catalyzed multicomponent coupling: synthesis of 1,3-oxazolidine derivatives using paraformaldehyde as a C1 feedstock†
SnCl2 catalyzed the three-component coupling of aniline, epoxide, and paraformaldehyde, resulting in the synthesis of 1,3-oxazolidine derivatives. The reaction is simple and does not require any additives, bases, or oxidants, and proceeds at moderate temperature with good functional group tolerance. The scope of the utilization of paraformaldehyde as the methylene source was further extended to the synthesis of benzothiazole and 4,4′-methylenebis(N,N-dimethylaniline) using the same catalyst. A catalytic pathway was proposed based on the control experiments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
The international home of synthetic, physical and biomolecular organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


