苯酚和噻吩酚催化氮丙啶的区域选择性布氏酸开环;获得功能化吲哚、吲哚、苯并噻嗪、二氢苯并噻嗪、苯并噁嗪和苯并色烯的途径。
IF 2.9
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
有报道称,苯酚和噻吩酚催化的卤代酸可实现氮丙啶类化合物的区域选择性开环。一系列氮丙啶类化合物与一系列酚类和噻吩酚类的结合使报告的方案具有通用性。在室温下于极短的时间内完成反应体现了所开发技术的独特性。为了强调所开发方法的应用性,这些产品已被用于进一步合成一系列有用的新型杂环分子,如吲哚、吲哚、苯并噻嗪、二氢苯并噻嗪、苯并噁嗪和苯并色烯。本文章由计算机程序翻译,如有差异,请以英文原文为准。
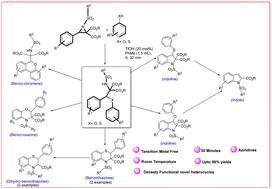
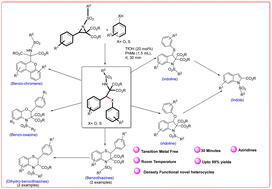
Regioselective Brønsted acid catalyzed ring opening of aziridines by phenols and thiophenols; a gateway to access functionalized indolines, indoles, benzothiazines, dihydrobenzo-thiazines, benzo-oxazines and benzochromenes†
Brønsted acid catalyzed regioselective ring opening of aziridines by phenols and thiophenols have been reported. Involvement of a series of aziridines with a range of phenols and thiophenols offer the generality of the reported protocol. Completion of the reaction at room temperature within very short time brings the uniqueness of the developed technique. To emphasis on the application of the developed methodology, the products have been used for the further synthesis of a range of useful and novel heterocyclic molecules such as indolines, indoles, benzothiazines, dihydrobenzothiazines, benzo-oxazines and benzochromenes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
The international home of synthetic, physical and biomolecular organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


