磷化氢促进分子内 Rauhut-Currier/Wittig 级联反应以获得(杂)炔融合二胍。
IF 2.9
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们描述了首个由膦促进的分子内 Rauhut-Currier 反应,该反应引发了分子内 Wittig 过程,从而组装出新类别的二喹烷。在无金属和中性条件下,这种一锅反应策略可随时获得简单的二喹啉和含有最多两个连续全碳季中心的各种(杂)烯融合二喹啉。我们展示了该方法在多种底物上的通用性,并证明了它在获得与天然产物合成和材料科学相关的各种高级中间体方面的合成效用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
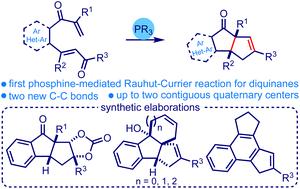
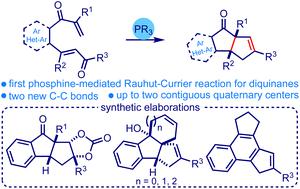
Phosphine-promoted intramolecular Rauhut–Currier/Wittig reaction cascade to access (hetero)arene-fused diquinanes†
We describe the first phosphine-promoted intramolecular Rauhut–Currier reaction that triggers an intramolecular Wittig process assembling new classes of diquinanes. The one-pot strategy provides ready access to simple diquinanes and various (hetero)arene-fused diquinanes incorporated with up to two contiguous all-carbon quaternary centers under metal-free and neutral conditions. We showcased the generality of the method on a broad range of substrates and demonstrated its synthetic utility in accessing various advanced intermediates relevant to natural product synthesis and material science.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
The international home of synthetic, physical and biomolecular organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


