使用 TBHP 作为氧化剂对 1,3-二羰基衍生物进行高选择性 C-N 和 C-S 双官能化。
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
通过一个给定的方案,描述了一种选择性合成 (E)-3-氨基-2-硫氰基-α,β-不饱和羰基化合物的直接电合成/无光催化剂、原子经济且高效的方法。本方法的特点是使用廉价的硫氰酸铵,以 TBHP 作为氧化剂,实现 1,3-二羰基化合物的双重官能化,为通过自由基过程选择性地形成 C-N 键和 C-S 键提供了一条快速而实用的途径。这种方法的底物范围广、收率高,而且可以进一步开发产品,以构建杂环化合物和其他官能团。本文章由计算机程序翻译,如有差异,请以英文原文为准。
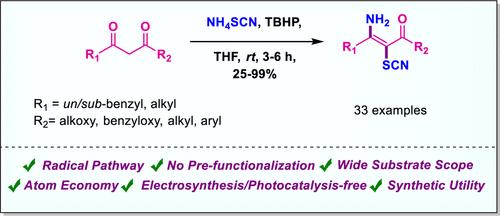
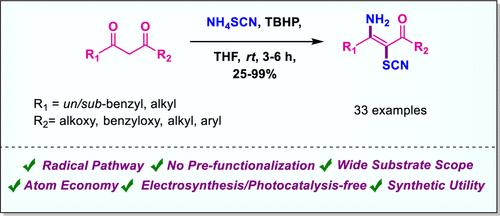
Highly Selective C–N and C–S Dual Functionalization of 1,3-Dicarbonyl Derivatives Using TBHP as an Oxidant
A direct electrosynthesis/photocatalyst-free, atom-economical, and efficient method for the selective synthesis of (E)-3-amino-2-thiocyanato-α,β-unsaturated carbonyl compounds is described through a given protocol. The present approach features the use of inexpensive ammonium thiocyanate to achieve dual functionalization of 1,3-dicarbonyl compounds using TBHP as an oxidant, providing a rapid and practical route to the selective formation of both C–N and C–S bonds via a radical process. This method offers a broad substrate scope with excellent yield and allows for further exploration of the products to construct heterocyclic compounds and other functionalities.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


