随机、双盲、安慰剂对照、平行组、多中心、IIA 期临床试验,评估每日口服 NFX88 治疗脊髓损伤患者神经病理性疼痛的安全性、耐受性和疗效。
IF 2.1
4区 医学
Q3 CLINICAL NEUROLOGY
引用次数: 0
摘要
研究设计双盲、随机、安慰剂对照、平行组多中心 IIA 期临床试验:评估慢性脊髓损伤(SCI)患者口服 NFX-88 的安全性和耐受性,并探讨其对疼痛控制的疗效:地点: 西班牙共 7 家脊髓损伤康复机构:方法:61 名外伤性完全或不完全脊髓损伤(C4-T12 水平)成人按 1:1:1:1 的比例随机接受安慰剂、NFX88 1.05 克、2.1 克、4.2 克/天的治疗,疗程长达 12 周。安慰剂或 NFX-88 作为原有普瑞巴林(每天 150-300 毫克)的附加疗法。对安全性和耐受性进行了评估,并以视觉模拟量表(VAS)作为主要测量指标,以探讨 NFX-88 在疼痛控制方面的疗效:结果:四个研究组均未报告与治疗相关的严重不良反应。44 名 SCI 患者完成了研究并接受了分析。从VAS分析和PainDETECT问卷(PD-Q)中获得的数据表明,在减轻SCI患者的神经病理性疼痛(NP)方面,NFX88与普瑞巴林的联合治疗比普瑞巴林与安慰剂的联合治疗更有效,而且2.10克/天的剂量能带来最显著的疼痛缓解:NFX88治疗安全性高,耐受性好,2.10克/天的剂量对缓解疼痛最有效。因此,这种首创的脂质介质具有良好的疗效,值得在未来的临床试验中进一步考虑。本文章由计算机程序翻译,如有差异,请以英文原文为准。
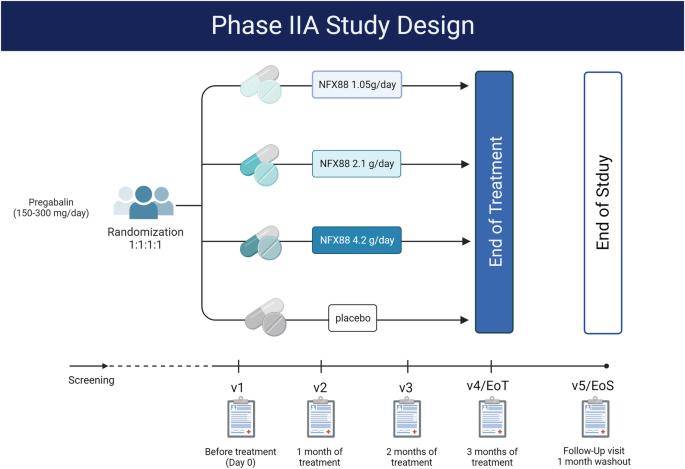
Randomised, double-blind, placebo-controlled, parallel-group, multicentric, phase IIA clinical trial for evaluating the safety, tolerability, and therapeutic efficacy of daily oral administration of NFX88 to treat neuropathic pain in individuals with spinal cord injury
Double-blind, randomized, placebo-controlled, parallel-group multicentric phase IIA clinical trial. To assess the safety and tolerability of oral administration of NFX-88 in subjects with chronic spinal cord injury (SCI) and explore its efficacy in pain control. A total of 7 spinal cord injury rehabilitation units in Spain. A total of 61 adult with traumatic complete or incomplete spinal cord injury (C4-T12 level), were randomised 1:1:1:1 to a placebo, NFX88 1.05 g, 2.1 g, 4.2 g/day for up to 12 weeks. The placebo or NFX-88 was administered as add-on therapy to pre-existing pregabalin (150–300 mg per day). Safety and tolerability were evaluated, and the Visual Analogue Scale (VAS) was the primary measure to explore the efficacy of NFX-88 in pain control. No severe treatment-related adverse effects were reported for any of the four study groups. 44 SCI individuals completed the study and were analysed. The data obtained from the VAS analysis and the PainDETECT Questionnaire (PD-Q) suggested that the combination of NFX88 with pregabalin is more effective than pregabalin with placebo at reducing neuropathic pain (NP) in individuals with SCI and that the dose 2.10 g/day causes the most dramatic pain relief. NFX88 treatment was found to be highly safe and well tolerated, with the dose of 2.10 g/day being the most effective at causing pain relief. Thus, the promising efficacy of this first-in-class lipid mediator deserves further consideration in future clinical trials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Spinal cord
医学-临床神经学
CiteScore
4.50
自引率
9.10%
发文量
142
审稿时长
2 months
期刊介绍:
Spinal Cord is a specialised, international journal that has been publishing spinal cord related manuscripts since 1963. It appears monthly, online and in print, and accepts contributions on spinal cord anatomy, physiology, management of injury and disease, and the quality of life and life circumstances of people with a spinal cord injury. Spinal Cord is multi-disciplinary and publishes contributions across the entire spectrum of research ranging from basic science to applied clinical research. It focuses on high quality original research, systematic reviews and narrative reviews.
Spinal Cord''s sister journal Spinal Cord Series and Cases: Clinical Management in Spinal Cord Disorders publishes high quality case reports, small case series, pilot and retrospective studies perspectives, Pulse survey articles, Point-couterpoint articles, correspondences and book reviews. It specialises in material that addresses all aspects of life for persons with spinal cord injuries or disorders. For more information, please see the aims and scope of Spinal Cord Series and Cases.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


